Question
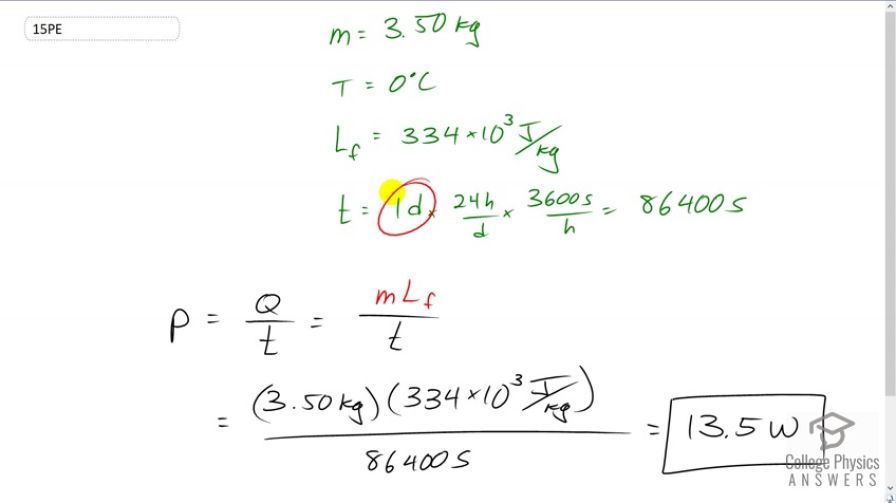
On a trip, you notice that a 3.50-kg bag of ice lasts an average of one day in your cooler. What is the average power in watts entering the ice if it starts at and completely melts to water in exactly one day? 1 watt = 1 joule / second (1W = 1 J/s).
Final Answer
Solution video
OpenStax College Physics, Chapter 14, Problem 15 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A cooler contains 3.50 kilograms of ice which has a temperature of zero degree Celsius and in exactly one day which we converted into seconds by multiplying by 24 hours per day and then 3600 seconds per hour. Ammm….. in that time we have all this ice melting and to a question is what is the rate of vartanity entering ice or the power transfer into the ice. So, that’s going to be the amount of energy required to melt that much ice divided by the time in which its melting. So, the amount of energy to melt ice will be the mass of ice multiply by the latent heat of fusion for water and then…... so, we have 3.5 kilograms times 334 times ten to three joules per kilogram divided by 86400 seconds. In this case 13.5 watts.
