Question
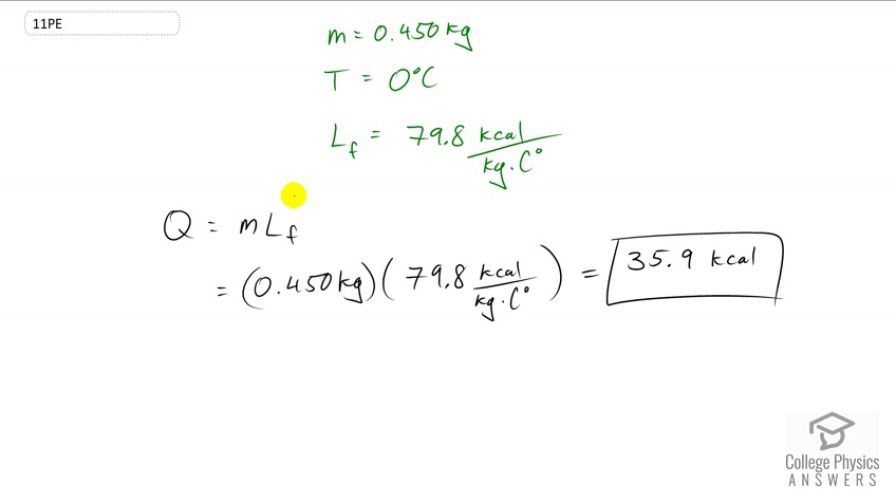
How much heat transfer (in kilocalories) is required to thaw a 0.450-kg package of frozen vegetables originally at if their heat of fusion is the same as that of water?
Final Answer
Solution video
OpenStax College Physics, Chapter 14, Problem 11 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. To change the state of frozen vegetables into water at a constant temperature, the amount of heat required will be just the latent heat of fusion. Fusion is maybe not the right word here because we're melting it, not fusing it, but it's the same thing whether you're making it solid or making it liquid. It's the same factor to use. So, the latent heat of fusion for water is 79.8 kilocalories per kilogram per Celsius degree. And then, we multiply that by the mass of 0.45 kilograms. And, this means the amount of heat required will be 35.9 kilocalories to melt the vegetables.
