Question
The formation of condensation on a glass of ice water causes the ice to melt faster than it would otherwise. If 8.00 g of condensation forms on a glass containing both water and 200 g of ice, how many grams of the ice will melt as a result? Assume no other heat transfer occurs.
Final Answer
Solution video
OpenStax College Physics, Chapter 14, Problem 14 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
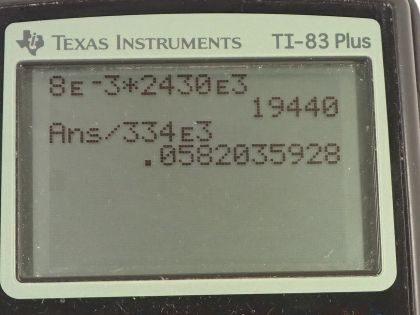
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. On a cold day, a glass full of water and some ice will cause vapor in the air to condense and when it condenses—changing phase from the vapor phase into liquid on the outside of the glass— it's going to release the latent heat of vaporization and doing so will heat up this ice cube in the glass, we assume, and the question is how much ice will melt as a result of this heat added by the condensation of the vapor? Okay. So we are told that 8.00 grams of water vapor condenses into water on the outside of the glass. So the amount of heat energy released by this condensation is the mass multiplied by the latent heat of vaporization. Now we use the latent heat of vaporization at 37.0 degrees Celsius as a better approximation than the value in this table; this table tells us what the latent heat of vaporization is at 100 degrees but the air surrounding this glass is not gonna be 100 degrees, it will be closer to 37.0 maybe... and the textbook tells us what this value is at 37.0 degrees— it's 2430 times 10 to the 3 joules per kilogram. So we multiply the mass by this latent heat of vaporization and get 1.9440 times 10 to the 4 joules of heat energy. And then we have to consider how much ice will melt as a result of this much heat energy being absorbed. So the heat energy will be the mass of the ice times the latent heat of fusion and so we can divide both sides by L f to get the amount of ice melting; it's gonna be Q divided by L f. So that's 1.9440 times 10 to the 4 joules of heat energy absorbed from the condensing water vapor divided by 334 times 10 to the 3 joules per kilogram— latent heat of fusion for water— and that's gonna be 58.2 grams. So that's a bit surprising isn't it that only 8.00 grams of water vapor condensing will cause many multiples more grams of ice to melt.