Question
Oxygen reaches the veinless cornea of the eye by diffusing through its tear layer, which is 0.500-mm thick. How long does it take the average oxygen molecule to do this?
Final Answer
2.1 min
Solution video
OpenStax College Physics, Chapter 12, Problem 64 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
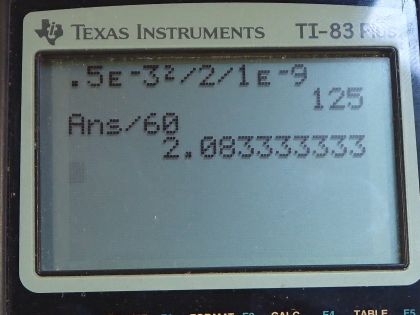
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We're going to determine how long it takes for the average oxygen molecule to diffuse from the tear layer into the eyes cornea. And we have that the distance that has to travel, we're told in this question is half of a millimeter, which is 0.5 times 10 to the minus three meters. And we look up with the diffusion constant is for an oxygen molecule in water, which is 1.0 times 10 to the minus nine meters squared per second. So the average distance that diffusing molecule will travel is square root of two times the diffusion constant multiplied by time and we square both sides. And we have X root mean squared, squared is two times diffusion constant times time. And we can divide both sides by two D to solve for t. So, the time then is 0.5 times 10 to the minus three meters squared divided by two times 1.0 times 10 to the minus nine meters squared per second and that's a 125 seconds which we convert into minutes. And that is 2.1 minutes on average.