Question
Approximately how many atoms thick is a cell membrane, assuming all atoms there average about twice the size of a hydrogen atom?
Final Answer
50 atoms
Solution video
OpenStax College Physics, Chapter 1, Problem 33 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
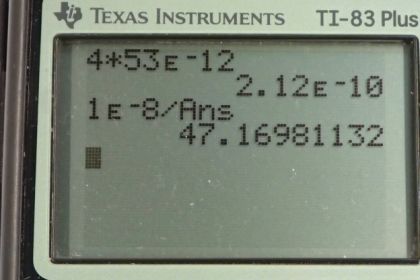
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko We're going to figure out how many atoms we can assume are stacked up to make the thickness of a cell membrane. So, we need to make assumptions about what the cell membrane thickness is and what the diameter of an atom is. So, the thickness of a cell membrane, when I googled it, it worked out to about 10 nanometers, which is... the nano prefix means 10 to the minus 9. So, we have 10 times 10 to the minus 9, which is 1 times 10 to the minus 8 meters. So, that's the size of our cell membrane. And we're told that the size of a typical atom that composes this membrane is 2 times the size of a hydrogen atom. So, I looked up the size of a hydrogen atom and there is a well known quantity called Bohr radius, which is the radius of a hydrogen atom because Neil Bohr calculated what that radius should be, and that was a big deal because it means he understood a lot to be able to make that calculation. So, we have 2 times this Bohr radius or hydrogen radius. And, well, 2 times 2 makes 4. So, we have the size of a typical atom in the cell membrane is 4 times the radius of a hydrogen atom. So, that's 4 times 53 picometers. And pico is a metric prefix meaning multiplied by 10 to the minus 12. And so, we have this working out to 2.12 times 10 to the minus 10 meters, is the size of a typical atom in the cell membrane. Now, of course, we don't deserve to have this much precision, but we'll keep it anyway because this is an intermediate calculation and so there is no need to round things yet. So, the size of our membrane is going to be some number times the size of the atoms that compose it and we’ll switch the sides around of this equation and we’ll divide both sides by the size of an atom. And then, we have x equals the size of the membrane divided by the size of an atom. So, that’s 1 times 10 to the minus 8 meters divided by 2.12 times 10 to the minus 10 meters, working out to 47.1698, which has no units because this is a factor. It's just a number that's getting multiplied by the other number. It's a counting number. It’s the number of atoms here. And so, since we're dealing with assumptions in our calculations, these quantities do not have much precision. And so, our answer should have only one significant figure. And so, we round this to 50 atoms, is the assumption for the number of atoms that make up the thickness of a cell membrane.