Question
What is the mass of in a cancer therapy transillumination unit containing 5.00 kCi of
Final Answer
Solution video
OpenStax College Physics, Chapter 32, Problem 20 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
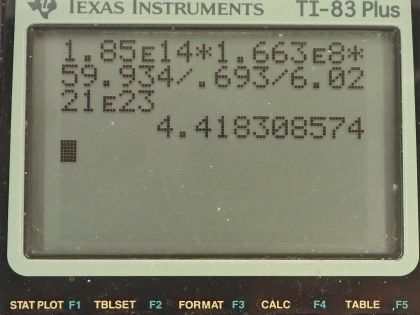
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We want to know the mass of cobalt-60 in a sample that has 5.00 kilocuries of activity. The half-life of cobalt-60 is 5.271 years, which I looked up in the appendix of the textbook and the molar mass of cobalt-60 is 59.934 grams per mol; I convert the half-life into seconds by multiplying by 365.25 days per year and then by 24 hours per day and 3600 seconds per hour and then converted the 5.00 kilocuries into becquerels by multiplying 5.00 times 10 to the 3 curies by 3.70 times 10 to the 10 becquerels per curie. Okay! So the activity is 0.693 multiplied by the number of atoms divided by the half-life and the number of atoms is the mass, which we are going to solve for divided by the molar mass multiplied by Avogadro's number so we substitute all this in for the number of atoms and then multiply top and bottom by this molar mass here so multiplied by molar mass of cobalt-60 on top and multiply by the molar mass of cobalt-60 on the bottom it cancels on the top and we are left with this expression here. This can be solved for the mass m by multiplying both sides by the half-life and multiply both sides by the molar mass and divide both sides by 0.693 and divide by Avogadro's number so we do that here and we do the same to the left side multiplying R by all of this as well. Then switch the sides around and we have mass then is the activity multiplied by half-life multiplied by molar mass divided by 0.693 times Avogadro's number. So we have 1.85 times 10 to the 14 becquerels—activity— by multiplying by 1.663 times 10 to the 8 seconds—half-life— times 59.934 grams per mol divided by 0.693 times Avogadro's number and we have 4.42 grams and we know the units are grams because this molar mass has units of grams in it.