Question
Verify that the total number of nucleons, total charge, and electron family number are conserved for each of the fusion reactions in the carbon cycle given in the above problem. (List the value of each of the conserved quantities before and after each of the reactions.)
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics, Chapter 32, Problem 38 (Problems & Exercises)
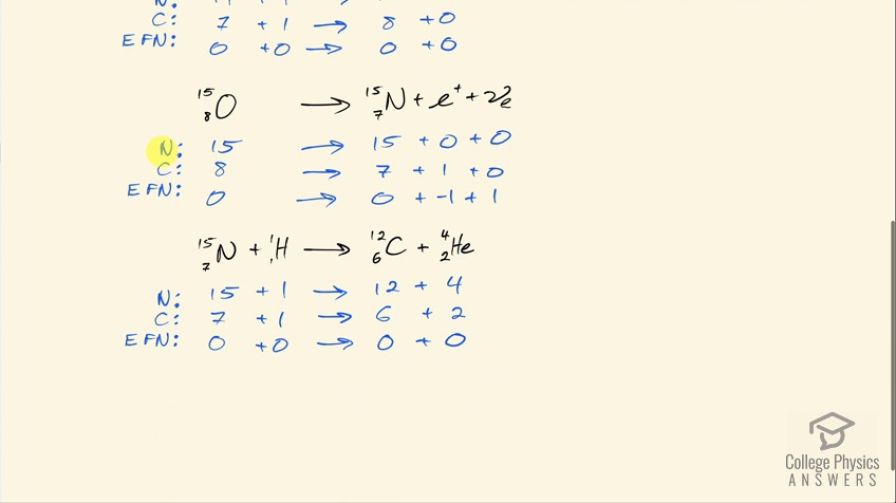
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. In the carbon fusion cycle— each step of which is written in black here— we are going to confirm that the number of nucleons the total charge and the electron family number is conserved on both sides of this reaction. So that means the total of each of these things has to be some number on the left and that has to total the same number on the right. So looking at the number of nucleons in this carbon-12, we have 12 nucleons there plus 1 nucleon in this proton, this hydrogen atom and then that turns into 13 nucleons in this nitrogen atom plus a gamma ray which has 0 nucleons so that's a total of 13 on the left and the right so that checks out. For the charge, we have 6 plus in the carbon 1 plus in the hydrogen and then 7 plus in the nitrogen so there's a total of 7 on each side and then there's 0 electron family number for all of these particles. For this step here, we have 13 nucleons in the nitrogen-13 and on the right-hand side, we have 13 nucleons in carbon-13 and then 0 in the positron and 0 in the electron-neutrino so there's a total of 13 on both sides. There's a charge of 7 in this nitrogen because the atomic number for nitrogen is 7— there are 7 protons— and then there are 6 protons in the carbon and then there's a plus 1 charge in this positron and so that's a total of 7 on the right as well. The electron family number is 0 on the left, on the right hand side, we have negative 1 for this anti-matter counterpart to an electron and then we have a positive 1 electron family number for the electron-neutrino so a total of 0 on the right side as on the left. In the next step, we have 13 nucleons in carbon-13 plus 1 in this hydrogen and that turns into 14 nucleons in the nitrogen-14 and 0 in the gamma particle so there's a total of 14 on both sides. There's a plus 6 charge in carbon, plus 1 charge in the proton and then plus 7 charge in the nitrogen and that's a total of 7 on both sides and then there's 0 electron family number for all those particles in the that step. And then we have 14 nucleons in the nitrogen, 1 in the hydrogen for a total of 15 and on the right hand side, we have 15 nucleons in the oxygen and 0 in the gamma particle so that's 15 on both sides plus 7 charge among the 7 protons in the nitrogen plus 1 from the hydrogen and that's a total of 8 and on the right hand side, we have 8 protons in the oxygen and 0 in the gamma particle so that's a total of 8 charge on both sides and there's 0 electron family number for all those particles in the next step and then we have 15 nucleons in oxygen-15 and then 15 nucleons in nitrogen-15 and then 0 nucleons in the positron or the electron-neutrino so there's a total of 15 on both sides For the charge, we have 8 in the oxygen, 7 in the nitrogen and plus 1 in this positron and so that's 8 on both sides and then for the electron family number, we have 0 on the left 0 for the nitrogen, negative 1 for the positron and positive 1 for the electron-neutrino for a total of 0 on the right-side as well. And then lastly, we have 15 nucleons in nitrogen-15, 1 in hydrogen and that's total of 16 and then on the right-side, we have 12 on the carbon-12 plus 4 in the helium nuclide and that's a total of 16 on the right as well. And for the charge, we have 7 in nitrogen, 1 in hydrogen for a total of 8 and on the right side, we have 6 in carbon and 2 in helium for a total of 8 as well and then there's 0 electron family numbers on all these particles. And there we go!