Question
Find the mass of that has an activity of .
Final Answer
Solution video
OpenStax College Physics, Chapter 32, Problem 15 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
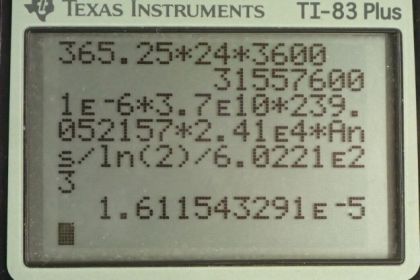
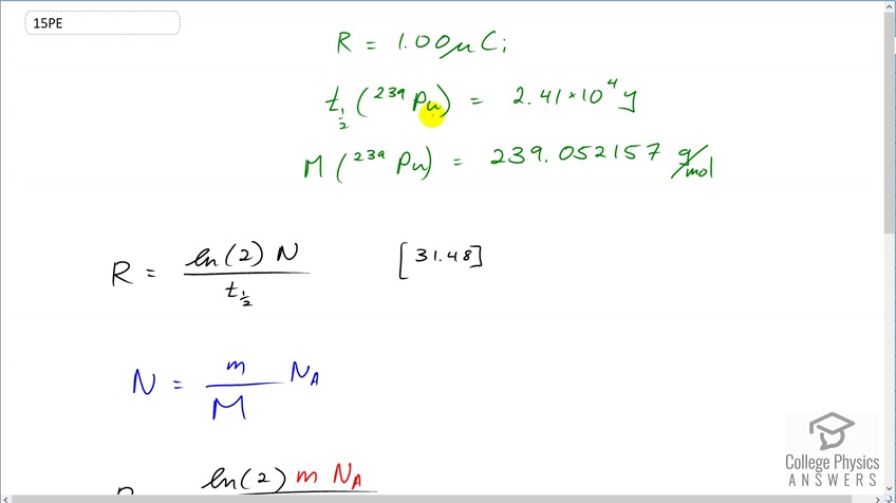
This is College Physics Answers with Shaun Dychko. We want to know the mass of plutonium-239 that has an activity of 1 microcurie. So the half-life of plutonium is 2.41 times 10 to the 4 years and it has an atomic mass of 239.052157 grams per mol and this is all data that we need and we looked up this information in appendix A of the textbook and we need this data to figure out what the mass of the plutonium is. So the activity from equation [31.48] is natural logarithm of 2 multiplied by the number of nuclei of plutonium-239 divided by the half-life. Now we want mass however so let's express this number of nuclei in terms of mass. And so we have the mass divided by the atomic mass gives us the number of mols of plutonium-239 and then we multiply that by Avogadro's number to convert mols into number of nuclei. So we substitute this in place of capital N here and then we solve for little m; we have to multiply both sides by the atomic mass times the half-life divided by natural logarithm of 2 times Avogadro's number. And we end up with mass is activity times atomic mass times half-life divided by natural logarithm of 2 times Avogadro's number and that's 1 microcurie converted into mks units which for activity is becquerels and so we have to convert the curies into becquerels and then multiply by the number of grams per mol for the plutonium-239 nuclide times by the half-life converted into mks units by multiplying by this many seconds per year and we divide by natural logarithm of 2 times Avogadro's number giving us 16.1 micrograms.