Question
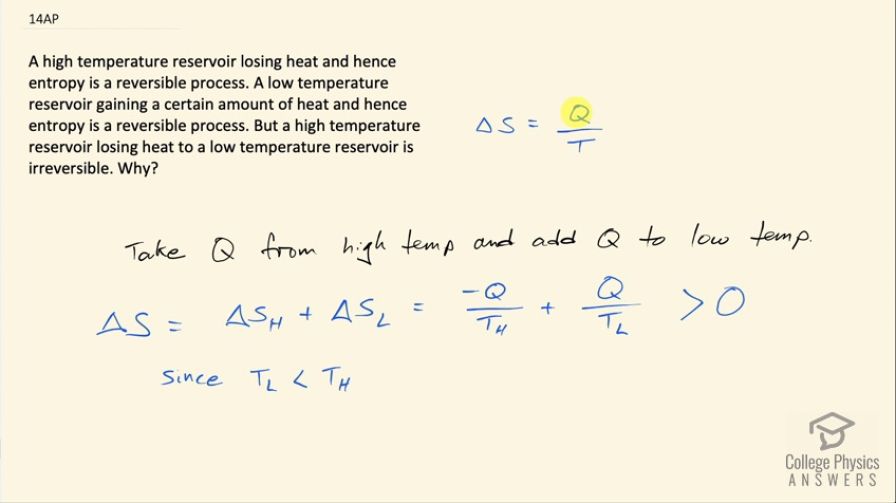
A high temperature reservoir losing heat and hence entropy is a reversible process. A low temperature reservoir gaining a certain amount of heat and hence entropy is a reversible process. But a high temperature reservoir losing heat to a low temperature reservoir is irreversible. Why?
Final Answer
Transferring heat from the high temperature reservoir to the low temperature reservoir will result in a greater gain in entropy in the low temperature reservoir than there is entropy reduction in the high temperature reservoir. This overall increase in entropy indicates that the process is irreversible.
Solution video
OpenStax College Physics for AP® Courses, Chapter 15, Problem 14 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. Change in entropy is the amount of heat transfer into or out of the heat reservoir divided by its temperature and so we are told that a high temperature reservoir losing heat to a low temperature reservoir is irreversible and the question is why? So if we can show that the total change in entropy is greater than zero then that means the process is irreversible. So the total change in entropy in this the scenario where the high temperature reservoir is losing some amount of heat Q to the low temperature reservoir, the total change in entropy is going to be negative Q, where Q I am taking to be the magnitude of heat transfer so taking it out of the high temperature reservoir makes it negative and then we are going to add that same amount of heat to the low temperature reservoir and this is going to be greater than zero—this sum— because this fraction here has Q divided by a small temperature whereas this fraction has this negative Q divided by a large temperature and so the quotient will be greater with a smaller denominator and so this positive term has greater magnitude than this negative term and so this sum is gonna be positive and since it's positive that means the process is irreversible.