Question
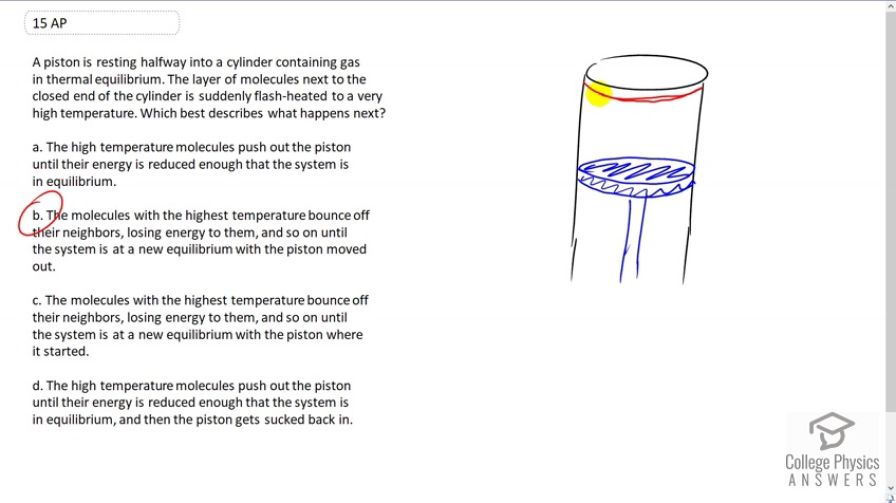
A piston is resting halfway into a cylinder containing gas in thermal equilibrium. The layer of molecules next to the closed end of the cylinder is suddenly flash-heated to a very high temperature. Which best describes what happens next?
- The high temperature molecules push out the piston until their energy is reduced enough that the system is in equilibrium.
- The molecules with the highest temperature bounce off their neighbors, losing energy to them, and so on until the system is at a new equilibrium with the piston moved out.
- The molecules with the highest temperature bounce off their neighbors, losing energy to them, and so on until the system is at a new equilibrium with the piston where it started.
- The high temperature molecules push out the piston until their energy is reduced enough that the system is in equilibrium, and then the piston gets sucked back in.
Final Answer
(b)
Solution video
OpenStax College Physics for AP® Courses, Chapter 15, Problem 15 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. There's a piston in the middle of a cylinder, and the gas molecules at the very top of the cylinder are ignited. And this is, basically it happens in a car engine because there are valves here that would suck in some gasoline, and it will get ignited up here. And these high-temperature gas molecules will bounce off each other and lose energy to their neighbors and so on until the systems at a new equilibrium, and the piston will have moved down because this gas will have expanded due to increased temperature. So the answer is B.