Question
A system does of work while of heat transfer occurs to the environment. What is the change in internal energy of the system assuming no other changes (such as in temperature or by the addition of fuel)?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 15, Problem 3 (Problems & Exercises)
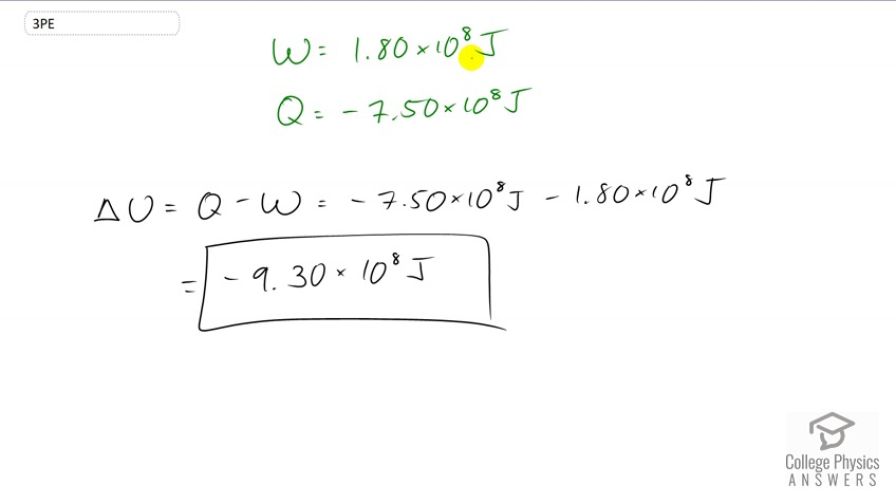
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We're told that a system does 1.8 times 10 to the 8 Joules of work and so that means W is going to be positive because it's the work done by the system. And the system also transfers a certain amount of heat energy to the environment and so this Q in our change in internal energy formula -- Q is going to be negative because this is heat transferred out of the system. So, we have change in internal energy is the heat added to the system minus the work done by the system. And the heat added is negative because it's actually losing energy, 7.5 times 10 to the 8 Joules. And then we'll subtract from that the work done by the system, 1.8 times 10 to the 8 Joules. And this gives negative 9.3 times 10 to the 8 Joules, is the change in internal energy.
