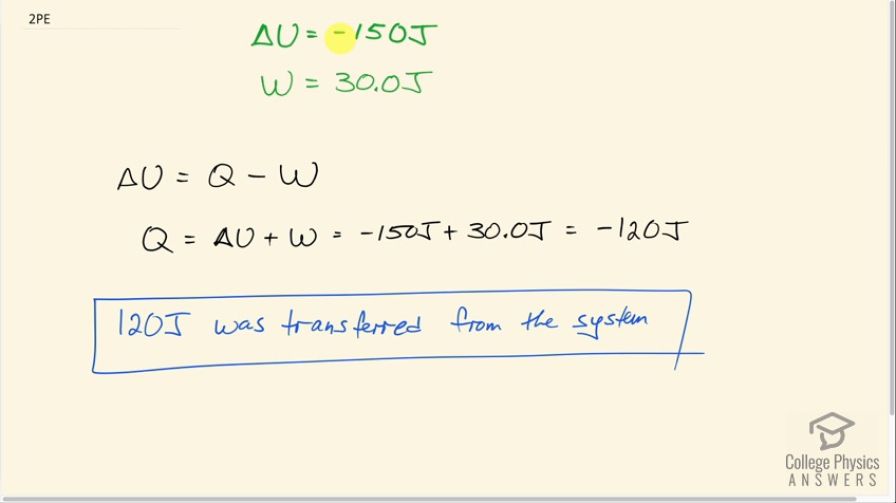
Question
How much heat transfer occurs from a system, if its internal energy decreased by 150 J while it was doing 30.0 J of work?
Final Answer
120 J was transferred from the system.
Solution video
OpenStax College Physics for AP® Courses, Chapter 15, Problem 2 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A system's internal energy decreases by 150 joules so that means ΔU is negative 150 joules— the negative representing a decrease in internal energy. The work done by the system or the work done on the environment is 30.0 joules and that's positive. So we have ΔU is the heat energy absorbed by the system so the heat transferred to the system minus the work done by the system. So we add w to both sides to solve for Q so Q is ΔU plus w so that's 150 joules plus 30.0 joules which is negative 120 joules and the negative sign here means that the heat was transferred out of the system because Q represents the amount of heat going into the system so being negative suggests that it's actually out of the system. So 120 joules was transferred from the system.
