Question
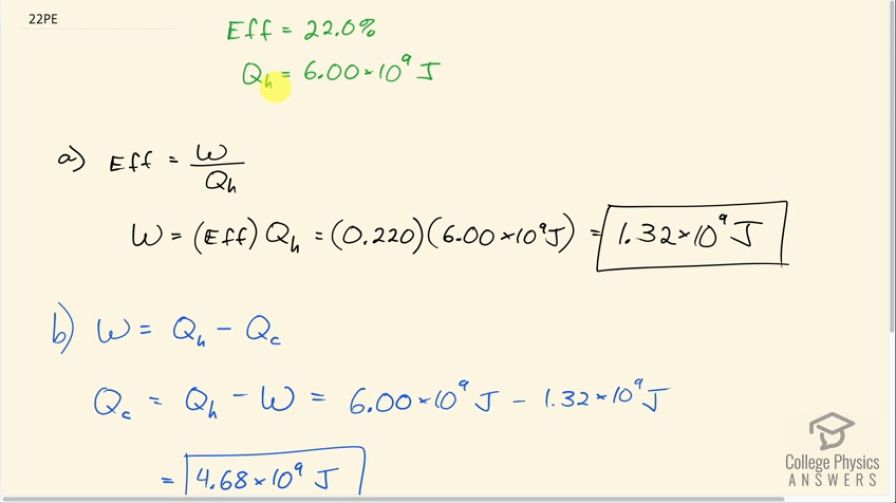
(a) What is the work output of a cyclical heat engine having a 22.0% efficiency and of heat transfer into the engine? (b) How much heat transfer occurs to the environment?
Final Answer
Solution video
OpenStax College Physics, Chapter 15, Problem 22 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A cyclical engine has an efficiency of 22.0 percent and it absorbs 6.00 times 10 to the 9 joules from the high temperature reservoir. Efficiency is the work done divided by the heat absorbed and we'll solve for w by multiplying both sides by Q h and we get that w then is efficiency times the amount of heat absorbed. So that's 0.220 times 6.00 times 10 to the 9 joules which is 1.32 times 10 to the 9 joules. Part (b) asks how much heat is expelled to the environment? Well the work done in a cyclical engine is the heat absorbed minus the heat expelled so we are solving for Q c here. So we'll add Q c to both sides and subtract w from both sides and we get then that Q c is Q h minus w. So that's 6.00 times 10 to the 9 joules of heat absorbed minus 1.32 times 10 to the 9 joules of work done which is 4.68 times 10 to the 9 joules of heat expelled to the environment.
