Question
What is the coefficient of performance of an ideal heat pump that has heat transfer from a cold temperature of to a hot temperature of ?
Final Answer
Solution video
OpenStax College Physics, Chapter 15, Problem 37 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
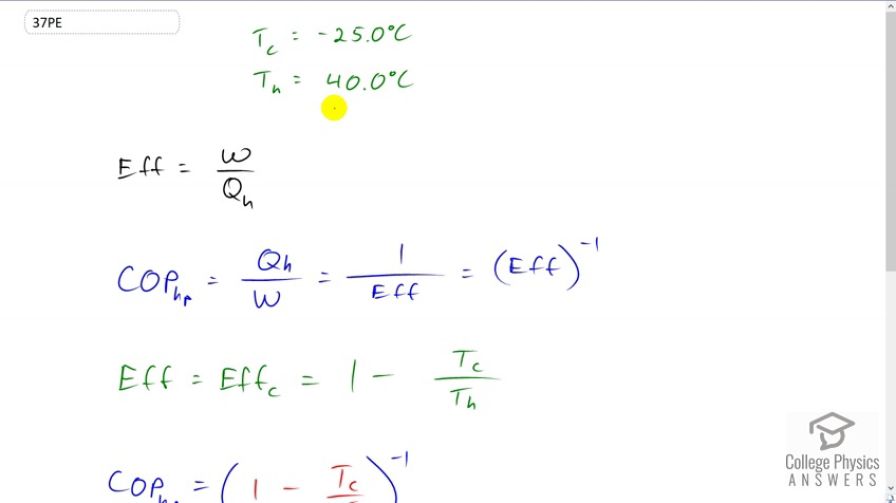
An ideal heat pump is operating between a cold reservoir temperature of -25 degrees Celsius and a hot reservoir temperature of 40 degrees Celsius. So, it's pulling heat from the cold and delivering it to the hot. And, the efficiency with which it does that is the work required to run the pump divided by how much heat is delivered to the hot reservoir. And, the coefficient of performance for a heat pump is considered Qh over W. So, it's the amount of heat delivered divided by the work done. And, we can see that this is the reciprocal of efficiency. And so, it's efficiency to the power of negative 1, in other words. And, we have a formula for the ideal efficiency, the Carnot efficiency in other words, which is 1 minus the cold reservoir temperature divided by the hot temperature. And so, we can plug this in for efficiency here, and so the coefficient of performance for an ideal heat pump 1 minus Tc over Th to the negative 1. So, that's 1 minus negative 25 degrees Celsius converted into Kelvin by adding 273.15 divided by 40 degrees Celsius converted into Kelvin, and then all to the negative 1, which is 4.82.
