Question
If you wish to detect details of the size of atoms (about ) with electromagnetic radiation, it must have a
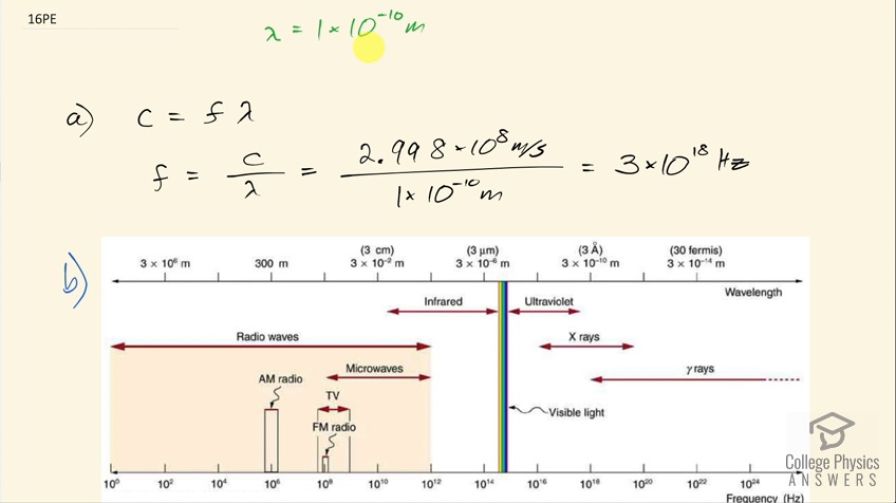
wavelength of about this size. (a) What is its frequency? (b) What type of electromagnetic radiation might this be?
Final Answer
- X rays
Solution video
OpenStax College Physics for AP® Courses, Chapter 24, Problem 16 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We need to choose a light with a frequency such that its wavelength is about the size of an atom, which is 1 times 10 to the minus 10 meters and the question is what is that frequency then? So the speed of light is the frequency multiplied by the wavelength and we can solve for f by dividing both sides by λ. So the frequency is 2.998 times 10 to the 8 meters per second divided by 1 times 10 to the minus 10 meters which is 3 times 10 to the 18 hertz. And part (b) is asking us what type of light is this? And so we look at this figure from the textbook and 10 to the 18 is the middle of the X-ray portion of the electromagnetic spectrum and so X-rays are what we will answer for part (b).
