Question
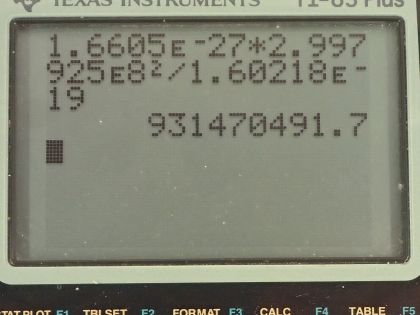
The unified atomic mass unit is defined to be . Verify that this amount of mass
converted to energy yields 931.5 MeV. Note that you must use four-digit or better values for and .
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 10 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to verify that 1 atomic mass unit which is the mass of 1.6605 times 10 to the minus 27 kilograms is equal to 931.5 megaelectron volts. So to verify that, we'll use this formula— mass times speed of light squared— to figure out how much energy is equivalent to this mass. So we take the mass multiplied by speed of light with lots of precision square that and then convert into electron volts by dividing by 1.60218 times 10 to the minus 19 joules— 1 electron volt for that many joules— and this works out to 931.5 megaelectron volts.