Question
has one of the longest known radioactive half-lives. In a difficult experiment, a researcher found that the activity of 1.00 kg of is 1.75 Bq. What is the half-life in years?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 52 (Problems & Exercises)
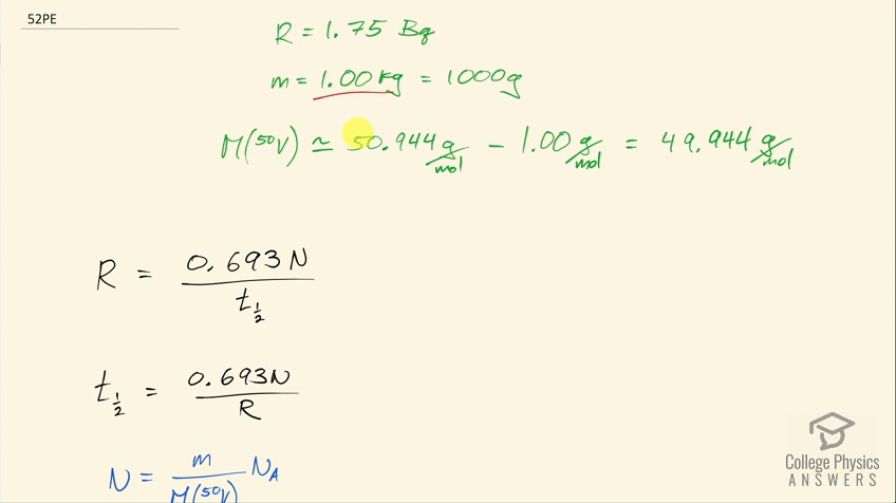
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. 1.00 kilogram of vanadium-50 has an activity of 1.75 becquerels. Now 1.00 kilogram, we're going to convert into grams—it's 1000 grams— because our molar mass is going to be in grams per mol. We look up the molar mass in the appendix here and we find vanadium-51 has a molar mass of 50.944; we don't have the molar mass of vanadium-50 listed in this appendix but we can guess approximately what it would be we can see that each nucleon adds approximately 1 gram per mol to each nuclide here. So if you go from 59 to 60 that takes you from 58.9 to 59.9 and that's, you know, true to the third decimal place here so it's a good estimate to say that one less neutron will reduce the molar mass by 1 gram per mol so anyway... molar mass of vandium-50 is about 49.944 grams per mol. Okay! So the activity is 0.693 times the number of atoms of vanadium-50 divided by its half-life and we can solve this for half-life by multiplying both sides by half-life divided by activity. So we get half-life then is 0.693 times the number of atoms divided by the activity. The number of atoms is going to be the mass divided by the molar mass multiplied by Avogadro's number so we substitute that in for N here and then you know clean things up by moving this to the denominator of the overall fraction here. So the half-life then is 0.693 times the mass times Avogadro's number divided by the activity times the molar mass. So that's 0.639 times 1000 grams times 6.0221 times 10 to the 23 atoms per mol divided 1.75 becquerels times 49.944 grams per mol and we'll get an answer in seconds because becquerels has units of reciprocal seconds and all the other units are canceling— the grams cancel with the grams, these reciprocal moles cancel with these reciprocal moles and we are left with 1 over 1 over s, which is s. Okay! We'll convert that into years by multiplying by 1 hour for every 3600 seconds and then by 1 day for every 24 hours and then by 1 year for every 365.25 days and we end up with 1.51 times 10 to the 17 years is the half-life of vanadium-50.
