Question
Mantles for gas lanterns contain thorium, because it forms an oxide that can survive being heated to incandescence for long periods of time. Natural thorium is almost 100% , with a half-life of . If an average lantern mantle contains 300 mg of thorium, what is its activity?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 48 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
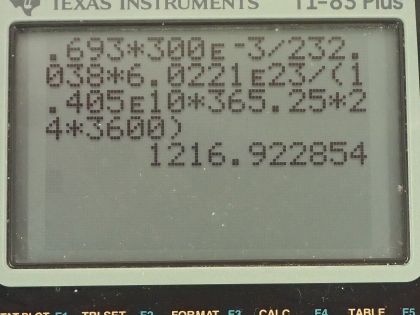
This is College Physics Answers with Shaun Dychko. We are going to find the activity of the mantle of a gas lantern. We are told that there is thorium-232 used to make the mantle— there is 300 milligrams of it, which is 300 times 10 to the minus 3 grams— the molar mass of thorium-232 I looked up in the appendix at the end of the textbook and it's 232.038 grams per mol and the half-life of thorium is 1.405 times 10 to the 10 years. Activity is equal to 0.693 multiplied by the number of atoms of thorium divided by its half-life and so that is 0.693 times 300 times 10 to the minus 3 grams converted into mols by multiplying by 1 mol for every 232.038 grams then we convert the mols into atoms by multiplying by Avogadro's number— 6.0221 times 10 to the 23 atoms per mol. So on the top we have number of atoms in this bracket here times 0.693 divided by the half-life converted into seconds in order to have units of becquerels in our answer. So we are multiplying by 1.405 times 10 to the 10 years by 365.25 days per year times 24 hours per day times 3600 seconds per hour and we are left with 1.22 times 10 to the 3 becquerels is the activity.