Question
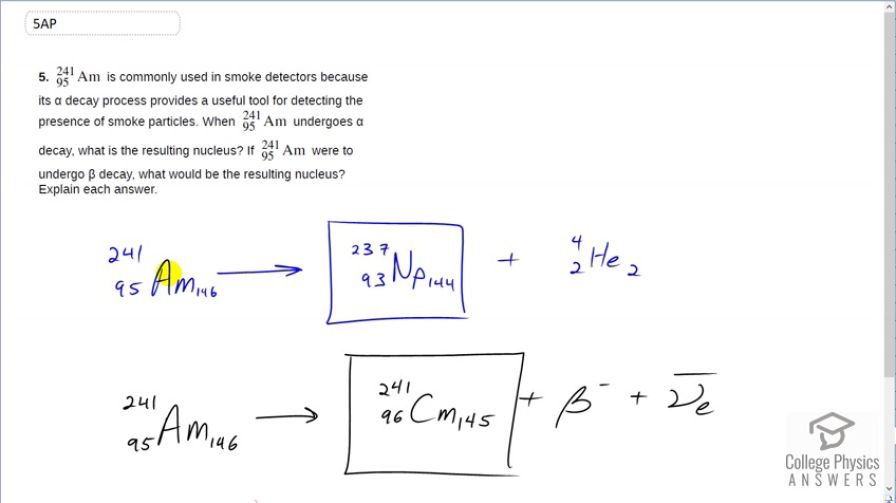
is commonly used in smoke detectors because its decay process provides a useful tool for detecting the presence of smoke particles. When undergoes decay, what is the resulting nucleus? If were to undergo decay, what would be the resulting nucleus? Explain each answer.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 5 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. Americium even though it is common in our modern world because it's in ionizing smoke detectors, it is very exotic this material because it's not naturally occurring, it's produced in nuclear reactions. So nevertheless, we have it everywhere. Americium-241 can convert into neptunium by way of α-decay and so this is the equation showing that and there are 237 nucleons in the neptunium plus the 4 in the α-particle totaling 241 that we started with. And the atomic number of americium decreases by 2 since 2 of the protons go to the α-particle leaving us with 93 leftover and 93 is the atomic number of neptunium. Then we are told to consider americium doing a β-decay. Well, if that happened then that would mean a neutron is converting into both a proton and an electron and in so doing it, increases the atomic number from 95 to 96 and the material with the atomic number 96 is called curium. And so we get americium turning into curium plus an electron plus an electron anti-neutrino in order to conserve electron family number.