Question
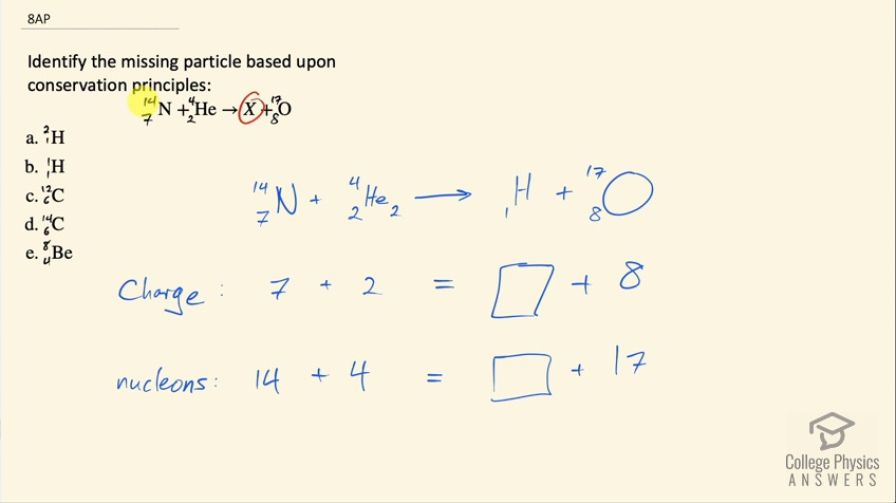
Identify the missing particle based upon conservation principles:
Final Answer
(b)
Solution video
OpenStax College Physics for AP® Courses, Chapter 31, Problem 8 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. Using conservation laws, we have to figure out what is this missing particle based on a combination of nitrogen-14 and helium-4 turning into something that we have to figure out plus oxygen-17? So our options are deuterium, which is hydrogen with 2 neutrons or regular hydrogen which has only 1 proton and carbon-12, carbon-14 or beryllium-8. Okay! So talking about conservation of charge, we have a charge of positive 7 in this nitrogen because of the 7 protons and two because of the two protons in the helium nucleus and that's going to turn into something that we don't know— let's pretend we don't know it's hydrogen— plus oxygen with 8 protons so 7 and 2 is 9 and we have 8 protons accounted for in the oxygen leaving 1 left over for this unknown thing, which we now know, is hydrogen. So now we need to figure out whether it's hydrogen with a neutron or hydrogen with only one proton. So then we look at the conservation of nucleons so we have 14 nucleons in the nitrogen plus 4 in the alpha particle for a total of 18 and we have 17 nucleons accounted for in the oxygen nucleus and so that leaves one nucleon left over for the hydrogen. So the answer is (b).