Question
What depth of mercury creates a pressure of 1.00 atm?
Final Answer
Solution video
OpenStax College Physics, Chapter 11, Problem 14 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
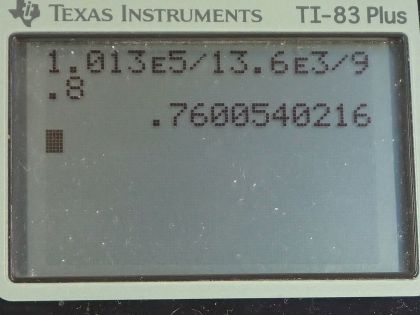
This is College Physics Answers with Shaun Dychko. Given the pressure exerted by a column of mercury of 1 atmosphere, we are gonna figure out what the height of that column of mercury is. So we know that the pressure equals that height times the mercury's density times gravitational field strength and we can solve this for h by dividing by ρg on both sides and we get that h is the pressure divided by density times gravitational field strength. So the one thing to be mindful of in this question is units. So we are given 1 atmosphere for the pressure but we have to convert that into pascals by multiplying by 1.013 times 10 to the 5 pascals per atmosphere and then the density for mercury we can look up in table [11.1] but we have two choices: we can say 13.6 grams per milliliter or we can say 13.6 times 10 to the 3 kilograms per cubic meter and it's the kilograms per cubic meter version that we are gonna choose here because we want mks units in a formula here that involves pascals because pascals are really newtons per square meter and so we need meters in this density here. Okay! So we have 1.013 times 10 to the 5 pascals divided by 13.6 times 10 to the 3 kilograms per cubic meter times 9.80 newtons per kilogram giving a height of 0.760 meters.