Question
A clock battery wears out after moving 10,000 C of charge through the clock at a rate of 0.500 mA. (a) How long did the clock run? (b) How many electrons per second flowed?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 20, Problem 10 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
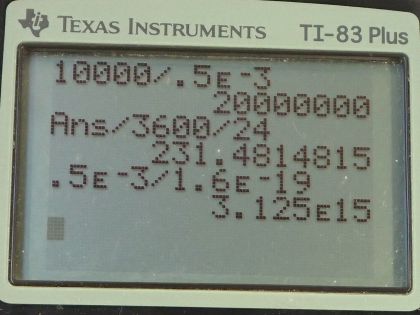
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A clock battery moves 10000 coulombs of charge through the clock circuit with a current of 0.500 milliamps, which is 0.500 times 10 to the minus 3 amps and the question in part (a) is how long do those batteries last? While the current is the amount of charge moved divided by the amount of time it takes to move the charge and we will solve this for t by multiplying both sides by t over I and so the time then is the amount of charge moved divided by the current. So that's 10000 coulombs divided by 0.500 times 10 to the minus 3 amps which is 2.000 times 10 to the 7 seconds which we convert into units that are more easy to relate to by multiplying by 1 hour for every 3600 seconds and then by 1 day for every 24 hours and we get 231 days. In part (b) we are asked how many electrons per second have moved through this circuit? So that's 0.500 times 10 to the minus 3 coulombs per second because coulombs per second is the abbreviation or sorry amps is the abbreviation for coulombs per second and then multiply this by 1 electron for every 1.60 times 10 to the minus 19 coulombs and we are left with electrons per second and that is 3.13 times 10 to the 15 electrons per second.