Question
Alkaline batteries have the advantage of putting out constant voltage until very nearly the end of their life. How long will an alkaline battery rated at and 1.58 V keep a 1.00-W flashlight bulb burning?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 20, Problem 54 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
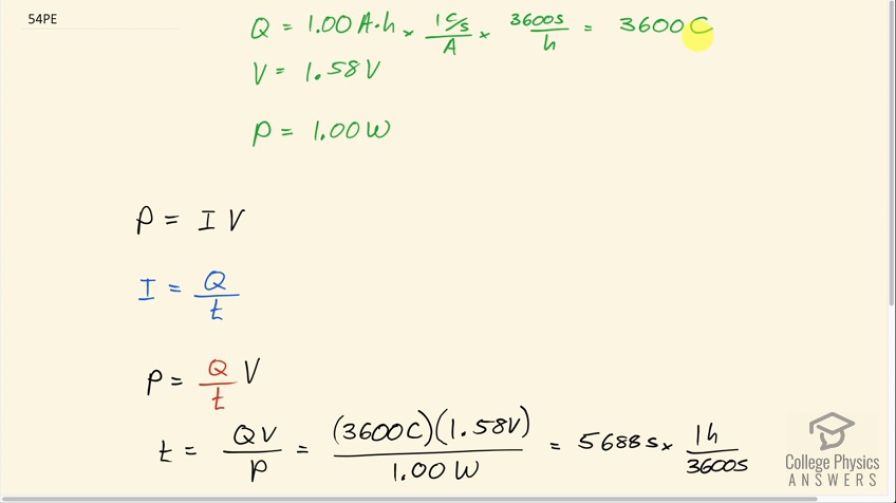
This is College Physics Answers with Shaun Dychko. An alkaline battery rated at 1.00 amp-hour has 3600 coulombs of charge available to flow through the flashlight circuit and we find that by multiplying by 1 coulomb per second for every amp and then by 3600 seconds per hour and we are left with coulombs in the end because the seconds cancel as well so it's 1 times 3600 coulombs. The voltage we are told is 1.58 volts and the power is 1.00 watt and we know that power equals current times voltage and we substitute for current by writing it as the rate of charge flow so Q divided by t in other words and here's the t factor that we are gonna solve for: we want to know how long will the battery keep the flashlight burning for? So we multiply both sides of this by t over power P and we get t then is QV over P. So that's 3600 coulombs times 1.58 volts divided by 1.00 watt, which is 5688 seconds and we will convert that into hours because that's an easier unit to think about because this is such a large number of seconds. So we divide by 3600 seconds and we end up with 1.58 hours is the total time this battery can keep the flashlight lit up for.
