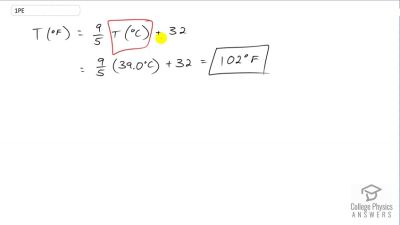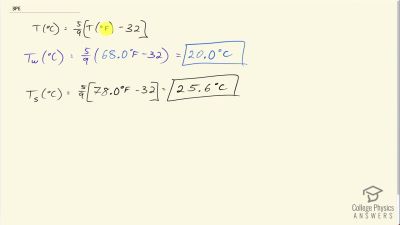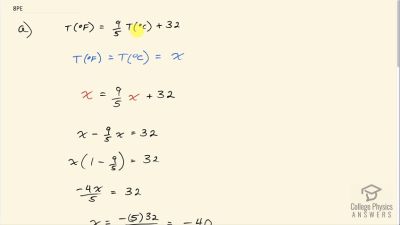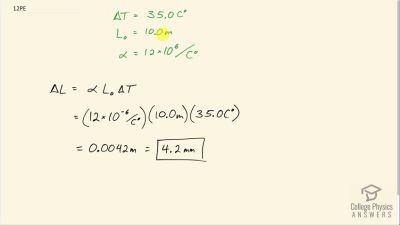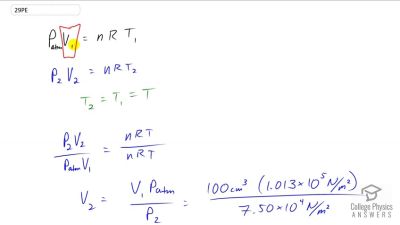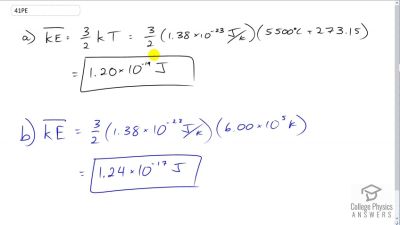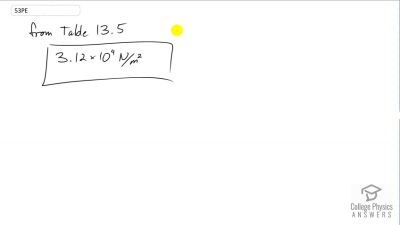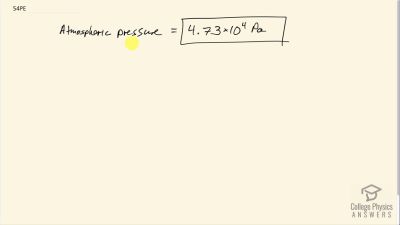Chapter 13
Chapter thumbnail

Chapter 13 : Temperature, Kinetic Theory, and the Gas Laws - all with Video Solutions
Problems & Exercises
Section 13.1: Temperature
Problem 2
Frost damage to most plants occurs at temperatures of or lower. What is this temperature on the Kelvin scale?
Problem 3
To conserve energy, room temperatures are kept at in the winter and in the summer. What are these temperatures on the Celsius scale?
Problem 4
A tungsten light bulb filament may operate at 2900 K. What is its Fahrenheit temperature? What is this on the Celsius scale?
Problem 5
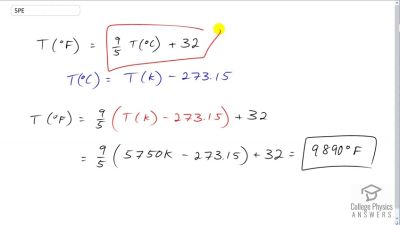
The surface temperature of the Sun is about 5750 K. What is this temperature on the Fahrenheit scale?
Problem 6
One of the hottest temperatures ever recorded on the surface of Earth was in Death Valley, CA. What is this temperature in Celsius degrees? What is this temperature in Kelvin?
Problem 7
(a) Suppose a cold front blows into your locale and drops the temperature by 40.0 Fahrenheit degrees. How many degrees Celsius does the temperature decrease when there is a decrease in temperature? (b) Show that any change in temperature in Fahrenheit degrees is nine-fifths the change in Celsius degrees.
Problem 8
(a) At what temperature do the Fahrenheit and Celsius scales have the same numerical value? (b) At what temperature do the Fahrenheit and Kelvin scales have the same numerical value?
Section 13.2: Thermal Expansion of Solids and Liquids
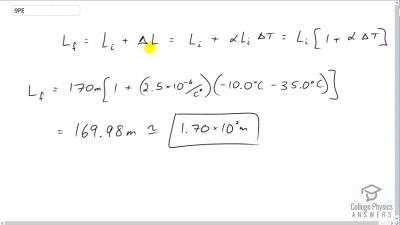
Problem 9
The height of the Washington Monument is measured to be 170 m on a day when the temperature is . What will its height be on a day when the temperature falls to ? Although the monument is made of limestone, assume that its thermal coefficient of expansion is the same as marble’s.
Problem 10
How much taller does the Eiffel Tower become at the end of a day when the temperature has increased by ? Its original height is 321 m and you can assume it is made of steel.
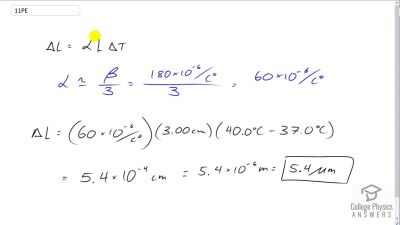
Problem 11
What is the change in length of a 3.00-cm-long column of mercury if its temperature changes from to , assuming the mercury is unconstrained?
Problem 12
How large an expansion gap should be left between steel railroad rails if they may reach a maximum temperature greater than when they were laid? Their original length is 10.0 m.
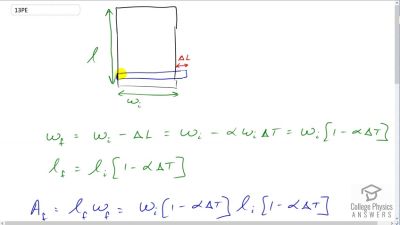
Problem 13
You are looking to purchase a small piece of land in Hong Kong. The price is “only” $60,000 per square meter! The land title says the dimensions are By how much would the total price change if you measured the parcel with a steel tape measure on a day when the temperature was above normal?
Problem 14
Global warming will produce rising sea levels partly due to melting ice caps but also due to the expansion of water as average ocean temperatures rise. To get some idea of the size of this effect, calculate the change in length of a column of water 1.00 km high for a temperature increase of .
Note that this calculation is only approximate because ocean warming is not uniform with depth.
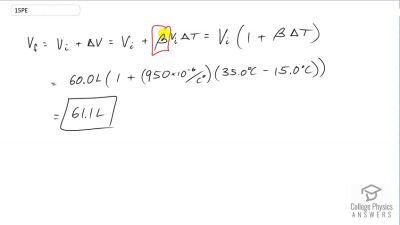
Problem 15
Show that 60.0 L of gasoline originally at will expand to 61.1 L when it warms to as claimed in Example 13.4.
Problem 16
(a) Suppose a meter stick made of steel and one made of invar (an alloy of iron and nickel) are the same length at . What is their difference in length at ? (b) Repeat the calculation for two 30.0-m-long surveyor’s tapes.
Problem 17
(a) If a 500-mL glass beaker is filled to the brim with ethyl alcohol at a temperature of how much will overflow when its temperature reaches ? (b) How much less water would overflow under the same conditions?
Problem 18
Most automobiles have a coolant reservoir to catch radiator fluid that may overflow when the engine is hot. A radiator is made of copper and is filled to its 16.0-L capacity when at . What volume of radiator fluid will overflow when the radiator and fluid reach their operating temperature, given that the fluid’s volume coefficient of
expansion is ? Note that this coefficient is approximate, because most car radiators have operating temperatures of greater than .
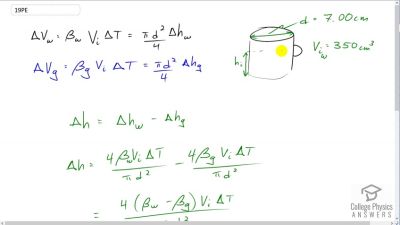
Problem 19
A physicist makes a cup of instant coffee and notices that, as the coffee cools, its level drops 3.00 mm in the glass cup. Show that this decrease cannot be due to thermal contraction by calculating the decrease in level if the of coffee is in a 7.00-cm-diameter cup and decreases in temperature from to (Most of the drop in level is actually due to escaping bubbles of air.)
Problem 20
(a) The density of water at is very nearly (it is actually ), whereas the density of ice at is . Calculate the pressure
necessary to keep ice from expanding when it freezes, neglecting the effect such a large pressure would have on the freezing temperature. (This problem gives you only an indication of how large the forces associated with freezing water might be.) (b) What are the implications of this result for biological cells that are frozen?
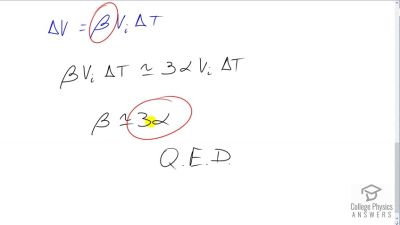
Problem 21
Show that , by calculating the change in volume of a cube with sides of length .
Section 13.3: The Ideal Gas Law
Problem 22
The gauge pressure in your car tires is at a temperature of when you drive it onto a ferry boat to Alaska. What is their gauge pressure later, when their temperature has dropped to ?
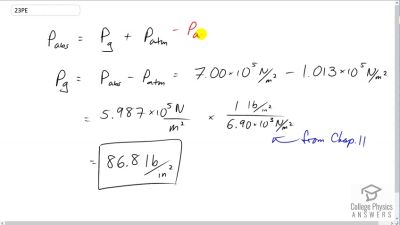
Problem 23
Convert an absolute pressure of to gauge pressure in . (This value was stated to be just less than in Example 13.9. Is it?)
Problem 24
Suppose a gas-filled incandescent light bulb is manufactured so that the gas inside the bulb is at atmospheric pressure when the bulb has a temperature of
. (a) Find the gauge pressure inside such a bulb when it is hot, assuming its average temperature is (an approximation) and neglecting any change in volume due to thermal expansion or gas leaks. (b) The actual final pressure for the light bulb will be less than calculated in part (a) because the glass bulb will expand. What will the actual final pressure be, taking this into account? Is this a negligible difference?
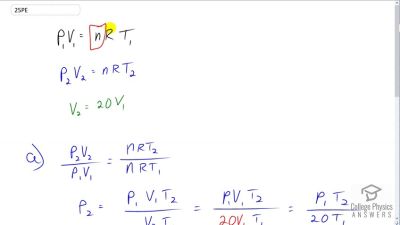
Problem 25
Large helium-filled balloons are used to lift scientific equipment to high altitudes. (a) What is the pressure inside such a balloon if it starts out at sea level with a temperature of and rises to an altitude where its volume is twenty times the original volume and its temperature is ?
(b) What is the gauge pressure? (Assume atmospheric pressure is constant.)
Problem 26
Confirm that the units of are those of energy for each value of : (a) , (b) , and (c) .
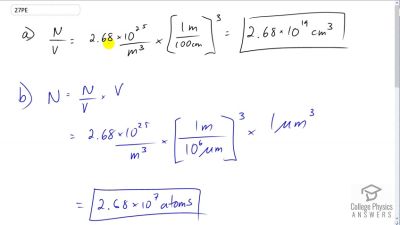
Problem 27
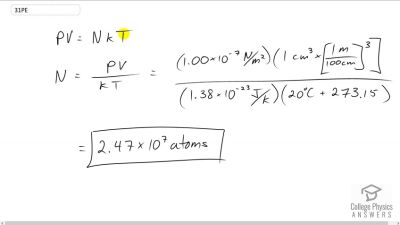
In the text, it was shown that for gas at STP. (a) Show that this quantity is equivalent to , as stated. (b) About how many atoms are there in one (a cubic micrometer) at STP? (c) What does your answer to part (b) imply about the separation of atoms and molecules?
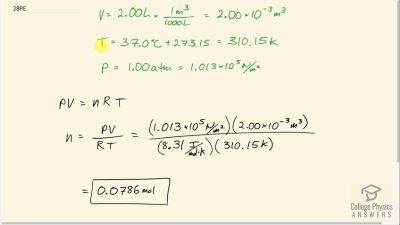
Problem 28
Calculate the number of moles in the 2.00-L volume of air in the lungs of the average person. Note that the air is at (body temperature).
Problem 29
An airplane passenger has of air in his stomach just before the plane takes off from a sea-level airport. What volume will the air have at cruising altitude if cabin pressure drops to ?
Problem 30
(a) What is the volume (in ) of Avogadro’s number of sand grains if each grain is a cube and has sides that are 1.0 mm long? (b) How many kilometers of beaches in length would this cover if the beach averages 100 m in width and 10.0 m in depth? Neglect air spaces between grains.
Problem 31
An expensive vacuum system can achieve a pressure as low as at . How many atoms are there in a cubic centimeter at this pressure and temperature?
Problem 32
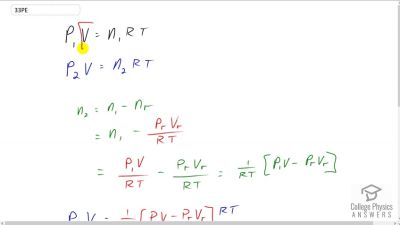
The number density of gas atoms at a certain location in the space above our planet is about , and the pressure is in this space. What is the temperature there?
Problem 33
A bicycle tire has a pressure of at a temperature of and contains of gas. What will its pressure be if you let out an amount of air that has a volume of at atmospheric pressure? Assume tire temperature and volume remain constant.
Problem 34
A high-pressure gas cylinder contains 50.0 L of toxic gas at a pressure of and a temperature of . Its valve leaks after the cylinder is dropped. The cylinder is cooled to dry ice temperature () to
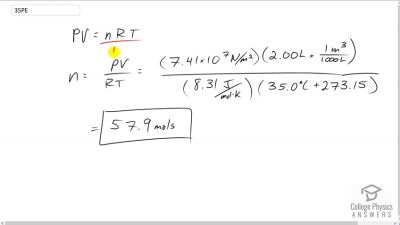
reduce the leak rate and pressure so that it can be safely repaired. (a) What is the final pressure in the tank, assuming a negligible amount of gas leaks while being cooled and that there is no phase change? (b) What is the final pressure if one-tenth of the gas escapes? (c) To what temperature must the tank be cooled to reduce the pressure to 1.00 atm (assuming the gas does not change phase and that there is no leakage during cooling)? (d) Does cooling the tank appear to be a practical solution?
Problem 36
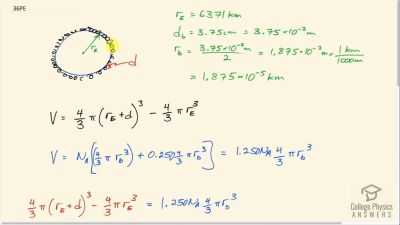
Calculate the depth to which Avogadro’s number of table tennis balls would cover Earth. Each ball has a diameter of 3.75 cm. Assume the space between balls adds an extra 25.0% to their volume and assume they are not crushed by their own weight.
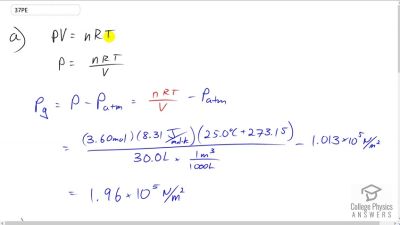
Problem 37
(a) What is the gauge pressure in a car tire containing 3.60 mol of gas in a 30.0 L volume? (b) What will its gauge pressure be if you add 1.00 L of gas originally at atmospheric pressure and ? Assume the temperature returns to and the volume remains constant.
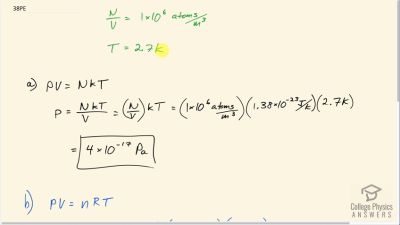
Problem 38
(a) In the deep space between galaxies, the density of atoms is as low as , and the temperature is a frigid 2.7 K. What is the pressure? (b) What volume (in ) is occupied by 1 mol of gas? (c) If this volume is a cube, what is the length of its sides in kilometers?
Section 13.4: Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature
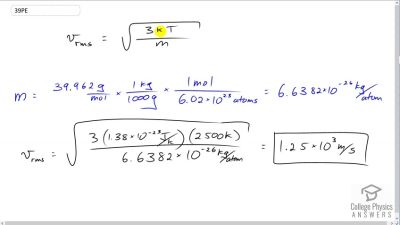
Problem 39
Some incandescent light bulbs are filled with argon gas. What is for argon atoms near the filament, assuming their temperature is 2500 K?
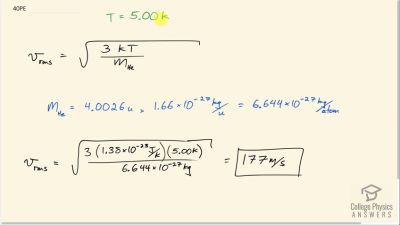
Problem 40
Average atomic and molecular speeds () are large, even at low temperatures. What is for helium atoms at 5.00 K, just one degree above helium’s liquefaction temperature?
Problem 41
(a) What is the average kinetic energy in joules of hydrogen atoms on the surface of the Sun? (b) What is the average kinetic energy of helium atoms in a region of the solar corona where the temperature is ?
Problem 42
The escape velocity of any object from Earth is 11.2 km/s. (a) Express this speed in m/s and km/h. (b) At what temperature would oxygen molecules (molecular mass is equal to 32.0 g/mol) have an average velocity equal to Earth’s escape velocity of 11.1 km/s?
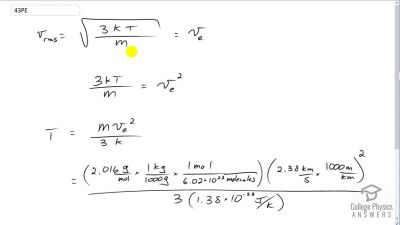
Problem 43
The escape velocity from the Moon is much smaller than from Earth and is only 2.38 km/s. At what temperature would hydrogen molecules (molecular mass is equal to 2.016 g/mol) have an average velocity equal to the Moon’s escape
velocity?
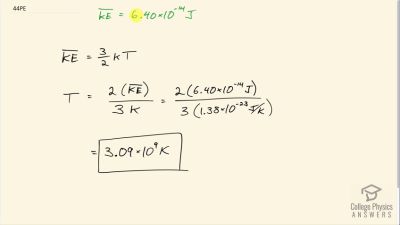
Problem 44
Nuclear fusion, the energy source of the Sun, hydrogen bombs, and fusion reactors, occurs much more readily when the average kinetic energy of the atoms is high—that is, at high temperatures. Suppose you want the atoms in your fusion experiment to have average kinetic energies of
. What temperature is needed?
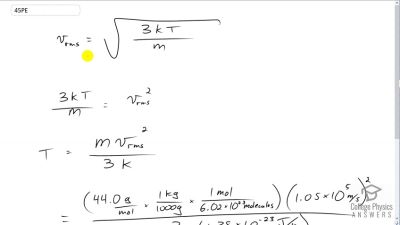
Problem 45
Suppose that the average velocity, , of carbon dioxide molecules (molecular mass is equal to 44.0 g/mol) in a flame is found to be . What temperature does this represent?
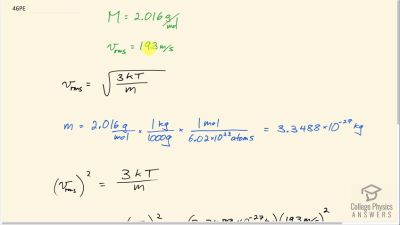
Problem 46
Hydrogen molecules (molecular mass is equal to 2.016 g/ mol) have an average velocity equal to 193 m/s. What is the temperature?
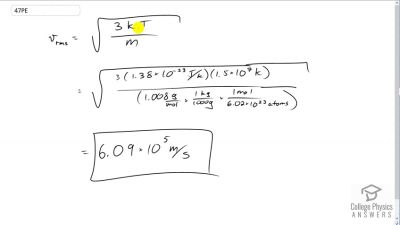
Problem 47
Much of the gas near the Sun is atomic hydrogen. Its temperature would have to be for the average velocity, to equal the escape velocity from the Sun. What is that velocity?
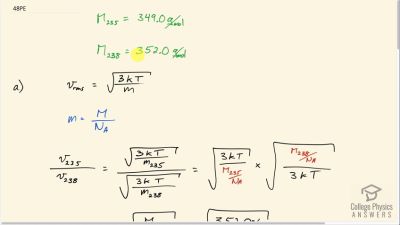
Problem 48
There are two important isotopes of uranium— and have different atomic masses. Only is very useful in nuclear reactors. One of the techniques for separating them (gas diffusion) is based on the different average velocities of uranium hexafluoride gas, . (a) The molecular masses for and are 349.0 g/mol and 352.0 g/mol, respectively. What is the ratio of their average velocities? (b) At what temperature would their average velocities differ by 1.00 m/s? (c) Do your answers in this problem imply that this technique may be difficult?
Section 13.6: Humidity, Evaporation, and Boiling
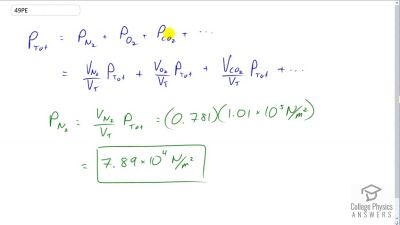
Problem 49
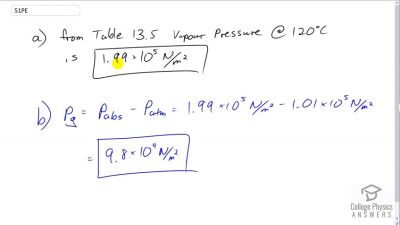
Dry air is 78.1% nitrogen. What is the partial pressure of nitrogen when the atmospheric pressure is ?
Problem 50
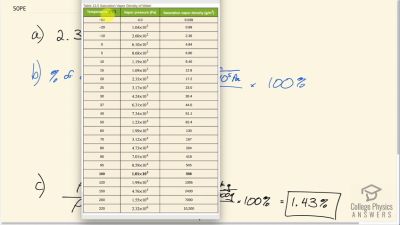
(a) What is the vapor pressure of water at ? (b) What percentage of atmospheric pressure does this correspond to? (c) What percent of air is water vapor if it has 100% relative humidity? (The density of dry air at is .)
Problem 51
Pressure cookers increase cooking speed by raising the boiling temperature of water above its value at atmospheric pressure. (a) What pressure is necessary to raise the boiling point to ? (b) What gauge pressure does this correspond to?
Problem 52
(a) At what temperature does water boil at an altitude of 1500 m (about 5000 ft) on a day when atmospheric pressure is ? (b) What about at an altitude of 3000 m (about 10,000 ft) when atmospheric pressure is ?
Problem 53
What is the atmospheric pressure on top of Mt. Everest on a day when water boils there at a temperature of ?
Problem 54
At a spot in the high Andes, water boils at , greatly reducing the cooking speed of potatoes, for example. What is atmospheric pressure at this location?
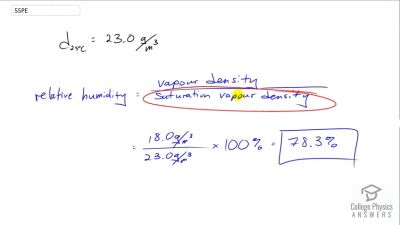
Problem 55
What is the relative humidity on a day when the air contains of water vapor?
Problem 56
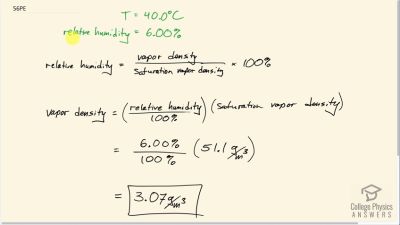
What is the density of water vapor in on a hot dry day in the desert when the temperature is and the relative humidity is 6.00%?
Problem 57
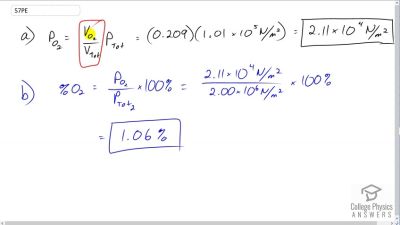
A deep-sea diver should breathe a gas mixture that has the same oxygen partial pressure as at sea level, where dry air contains 20.9% oxygen and has a total pressure of . (a) What is the partial pressure of oxygen at sea level? (b) If the diver breathes a gas mixture at a pressure of , what percent oxygen should it be to have the same oxygen partial pressure as at sea level?
Problem 58
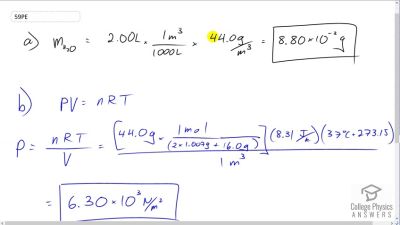
The vapor pressure of water at is . Using the ideal gas law, calculate the density of water vapor in that creates a partial pressure equal to this vapor pressure. The result should be the same as the saturation vapor density at that temperature ().
Problem 59
Air in human lungs has a temperature of and a
saturation vapor density of . (a) If 2.00 L of air is
exhaled and very dry air inhaled, what is the maximum loss of water vapor by the person? (b) Calculate the partial pressure of water vapor having this density, and compare it with the vapor pressure of .
Problem 60
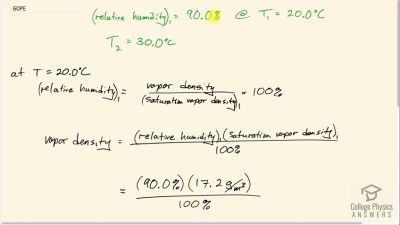
If the relative humidity is 90.0% on a muggy summer morning when the temperature is , what will it be later in the day when the temperature is , assuming the water vapor density remains constant?
Problem 61
Late on an autumn day, the relative humidity is 45.0% and the temperature is . What will the relative humidity be that evening when the temperature has dropped to , assuming constant water vapor density?
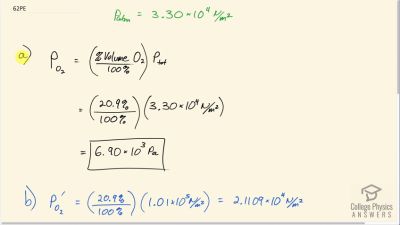
Problem 62
Atmospheric pressure atop Mt. Everest is . (a) What is the partial pressure of oxygen there if it is 20.9% of the air? (b) What percent oxygen should a mountain climber breathe so that its partial pressure is the same as at sea level, where atmospheric pressure is
? (c) One of the most severe problems for those climbing very high mountains is the extreme drying of breathing passages. Why does this drying occur?
Problem 63
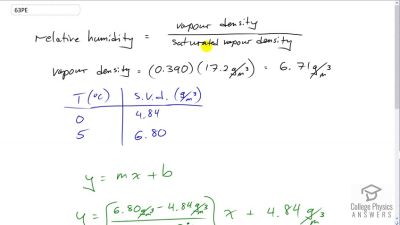
What is the dew point (the temperature at which 100% relative humidity would occur) on a day when relative humidity is 39.0% at a temperature of ?
Problem 64
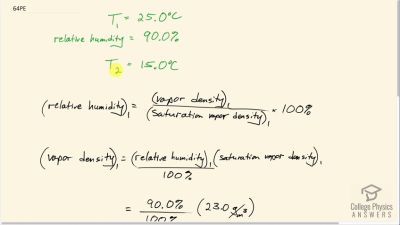
On a certain day, the temperature is and the relative humidity is 90.0%. How many grams of water must condense out of each cubic meter of air if the temperature falls to ? Such a drop in temperature can, thus, produce heavy dew or fog.
Problem 65
The boiling point of water increases with depth because pressure increases with depth. At what depth will fresh water have a boiling point of , if the surface of the water is at sea level?
Problem 66
(a) At what depth in fresh water is the critical pressure of water reached, given that the surface is at sea level? (b) At what temperature will this water boil? (c) Is a significantly higher temperature needed to boil water at a greater depth?
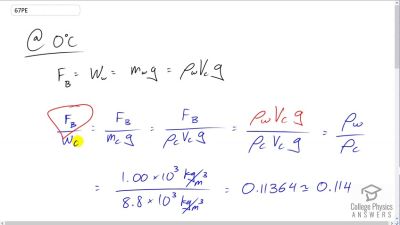
Problem 67
To get an idea of the small effect that temperature has on Archimedes’ principle, calculate the fraction of a copper block’s weight that is supported by the buoyant force in water and compare this fraction with the fraction supported in water.
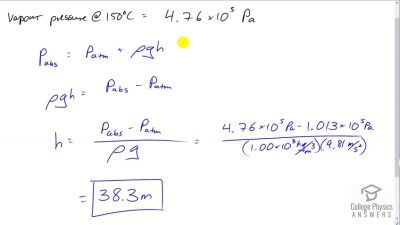
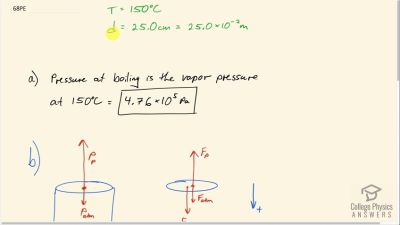
Problem 68
If you want to cook in water at , you need a pressure
cooker that can withstand the necessary pressure. (a) What pressure is required for the boiling point of water to be this high? (b) If the lid of the pressure cooker is a disk 25.0 cm in diameter, what force must it be able to withstand at this pressure?
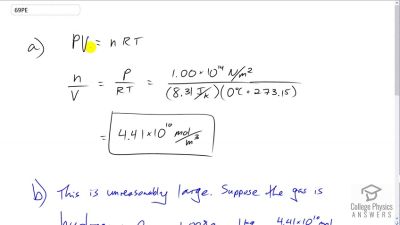
Problem 69
(a) How many moles per cubic meter of an ideal gas are there at a pressure of and at ? (b) What
is unreasonable about this result? (c) Which premise or assumption is responsible?
Problem 70
(a) An automobile mechanic claims that an aluminum rod fits loosely into its hole on an aluminum engine block because the engine is hot and the rod is cold. If the hole is 10.0% bigger in diameter than the rod, at what temperature will the rod be the same size as the hole? (b) What is unreasonable about this temperature? (c) Which premise is responsible?
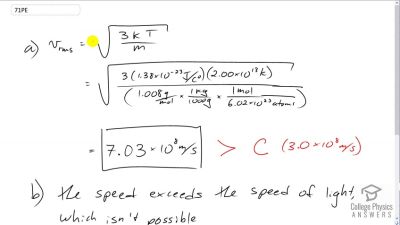
Problem 71
The temperature inside a supernova explosion is said to be . (a) What would the average velocity of hydrogen atoms be? (b) What is unreasonable about this velocity? (c) Which premise or assumption is responsible?
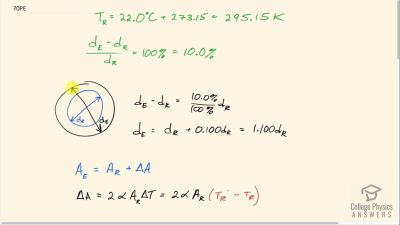
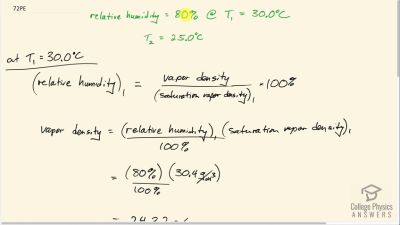
Problem 72
Suppose the relative humidity is 80% on a day when the temperature is . (a) What will the relative humidity be if the air cools to and the vapor density remains constant? (b) What is unreasonable about this result? (c) Which premise is responsible?
Test Prep for AP® Courses
Section 13.3: The Ideal Gas Law
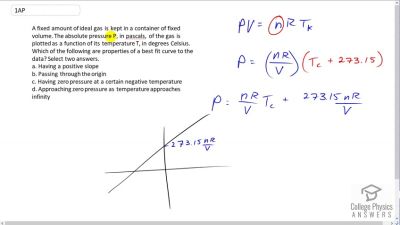
Problem 1 (AP)
A fixed amount of ideal gas is kept in a container of fixed volume. The absolute pressure P, in pascals, of the gas is plotted as a function of its temperature T, in degrees Celsius. Which of the following are properties of a best fit curve to the data? Select two answers.
- Having a positive slope
- Passing through the origin
- Having zero pressure at a certain negative temperature
- Approaching zero pressure as temperature approaches infinity
Problem 2 (AP)
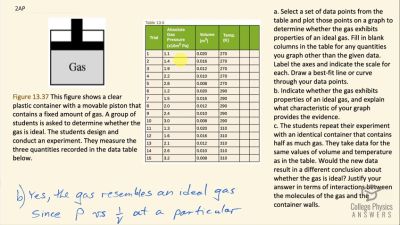
- Select a set of data points from the table below and plot those points on a graph to determine whether the gas exhibits properties of an ideal gas. Fill in blank columns in the table for any quantities you graph other than the given data. Label the axes and indicate the scale for each. Draw a best-fit line or curve through your data points.
- Indicate whether the gas exhibits properties of an ideal gas, and explain what characteristic of your graph provides the evidence.
- The students repeat their experiment with an identical container that contains half as much gas. They take data for the same values of volume and temperature as in the table. Would the new data result in a different conclusion about whether the gas is ideal? Justify your answer in terms of interactions between the molecules of the gas and the container walls.
Section 13.4: Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature
Problem 3 (AP)
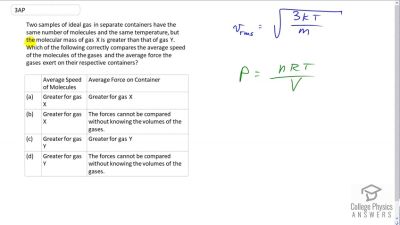
Two samples of ideal gas in separate containers have the same number of molecules and the same temperature, but the molecular mass of gas X is greater than that of gas Y. Which of the following correctly compares the average speed of the molecules of the gases and the average force the gases exert on their respective containers?
| Average Speed of Molecules | Average Force on Container | |
|---|---|---|
| (a) | Greater for gas X | Greater for gas X |
| (b) | Greater for gas X | The forces cannot be compared without knowing the volumes of the gases. |
| (c) | Greater for gas Y | Greater for gas Y |
| (d) | Greater for gas Y | The forces cannot be compared without knowing the volumes of the gases. |
Problem 4 (AP)
How will the average kinetic energy of a gas molecule change if its temperature is increased from to ?
- It will become sixteen times its original value.
- It will become four times its original value
- It will become double its original value
- It will remain unchanged.
Problem 5 (AP)
Figure 13.38 below is a graph that shows the Maxwell-Boltzmann distribution of molecular speeds in an ideal gas for two temperatures, T1 and T2. Which of the following statements is false?
- T1 is lower than T2
- The rms speed at T1 is higher than that at T2.
- The peak of each graph shows the most probable speed at the corresponding temperature.
- None of the above.
Problem 6 (AP)
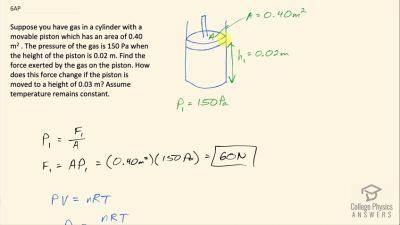
Suppose you have gas in a cylinder with a movable piston which has an area of . The pressure of the gas is 150 Pa when the height of the piston is 0.02 m. Find the force exerted by the gas on the piston. How does this force change if the piston is moved to a height of 0.03 m? Assume temperature remains constant.
Problem 7 (AP)
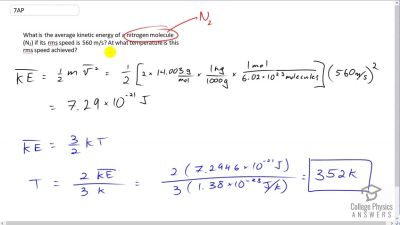
What is the average kinetic energy of a nitrogen molecule () if its rms speed is 560 m/s? At what temperature is this rms speed achieved?
Problem 8 (AP)
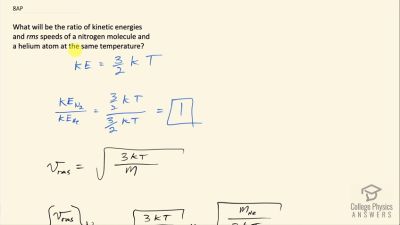
What will be the ratio of kinetic energies and rms speeds of a nitrogen molecule and a helium atom at the same temperature?