Question
How will the average kinetic energy of a gas molecule change if its temperature is increased from to ?
- It will become sixteen times its original value.
- It will become four times its original value
- It will become double its original value
- It will remain unchanged.
Final Answer
(c)
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 4 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
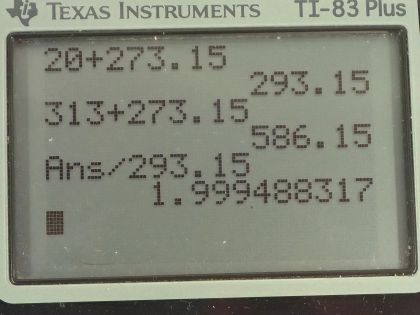
This is College Physics Answers with Shaun Dychko. How will the average kinetic energy of a gas molecule change as its temperature goes from 20 degrees Celsius to 313 degrees Celsius? Well, the kinetic energy formula says that it's 3 over 2 times Boltzmann's constant times the temperature but the temperature has to be in Kelvin— we need absolute temperature in our formulas— and so we take the initial temperature of 20 degrees Celsius, add 273.15 to it and we get 293.15 Kelvin. The final temperature is 313 degrees Celsius plus this number giving us 586.15 Kelvin. So the ratio of the kinetic energy final to initial is 3 over 2 times Boltzmann's constant times final temperature divided by 3 over 2 times k times initial temperature and these cancel leaving us with T f over T i. So 586.15 Kelvin divided by 293.15 Kelvin is about 2. So the kinetic energy will become double its original value; the answer is (c).