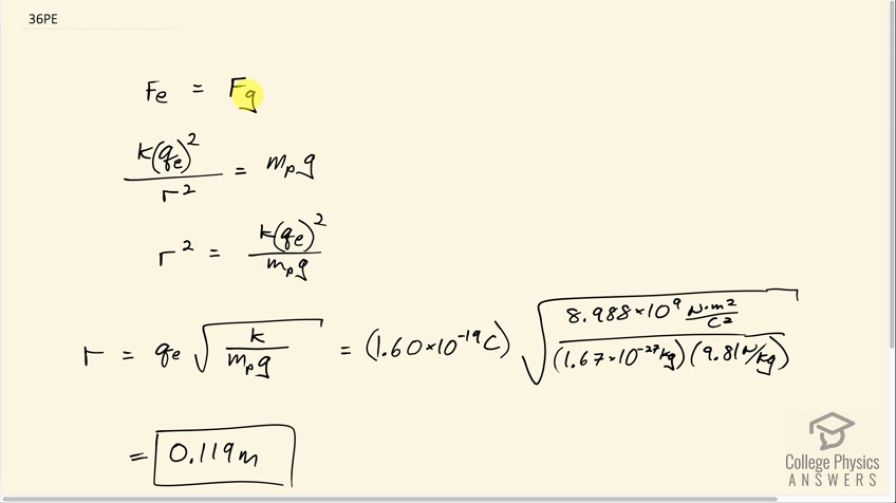
Question
At what distance is the electrostatic force between two protons equal to the weight of one proton?
Final Answer
Solution video
OpenStax College Physics, Chapter 18, Problem 22 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We want to know at what distance the electrostatic repulsion between two protons will equal the weight of one proton? So the weight of one proton is the mass of a proton multiplied by the gravitational field strength, g, and the electrostatic repulsion between two protons will be Coulomb's constant multiplied by the elementary charge and we'll do that multiplication twice because there are two protons and divide by the distance between them r squared and we are gonna have to solve for r eventually. So first we'll solve for r squared by multiplying both sides by r squared over mass of a proton times g and then we'll be left with this line here after we switch the sides around. So r squared equals k times elementary charge squared over mass of a proton times g and then take the square root of both sides and we have the distance between the protons is the elementary charge times square root k over mass—m p—times g. So that's 1.60 times 10 to the minus 19 coulombs times square root of Coulomb's constant divided by the mass of a proton which you have to look up... maybe you could use Google or something... and then multiply by gravitational field strength— 9.81 newtons per kilogram— and you get a distance of 0.119 meters.

