Question
(a) Using data from Table 7.1, calculate the mass converted to energy by the fission of 1.00 kg of uranium. (b) What is the ratio of mass destroyed to the original mass, ?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 28, Problem 48 (Problems & Exercises)
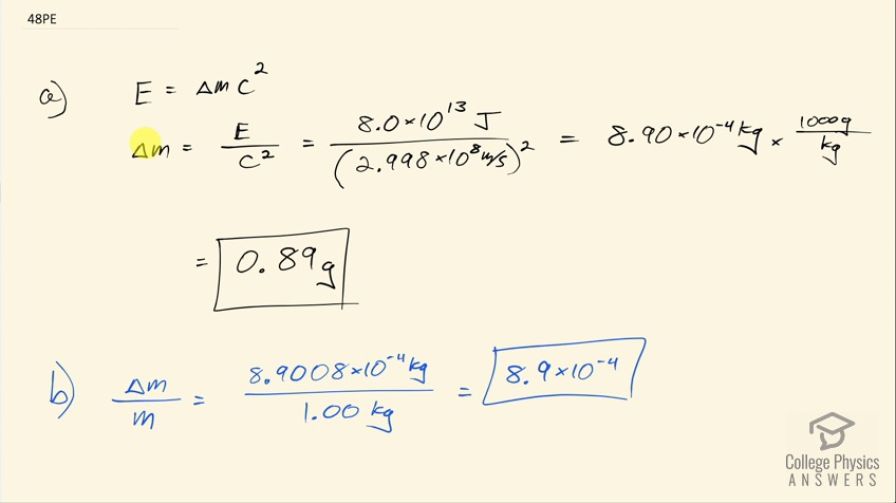
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to calculate how much mass of uranium is converted into energy when a kilogram of uranium undergoes fission. So we need to look up in table [7.1] how much energy is created in the fission of 1 kilogram of uranium— that's 8.0 times 10 to the 13 joules. So we have rearranged this formula: energy equals the mass defect times c squared; this Δm, this amount of mass that's converted directly into energy is called the mass defect often. We'll divide both sides by c squared and so this mass converted into energy is the energy produced divided by speed of light squared. So that's 8.0 times 10 to the 13 joules divided by 2.998 times 10 to the 8 meters per second squared and that's 8.90 times 10 to the minus 4 kilograms, which converted into grams is 0.89 grams. And this amount of mass converted into energy as a ratio of the total mass of uranium that we started with is this mass converted into energy divided by 1.00 kilogram that we started with and that's 8.9 times 10 to the minus 4.
