Question
What is the ratio of the average distances that oxygen will diffuse in a given time in air and water? Why is this distance less in water (equivalently, why is less in water)?
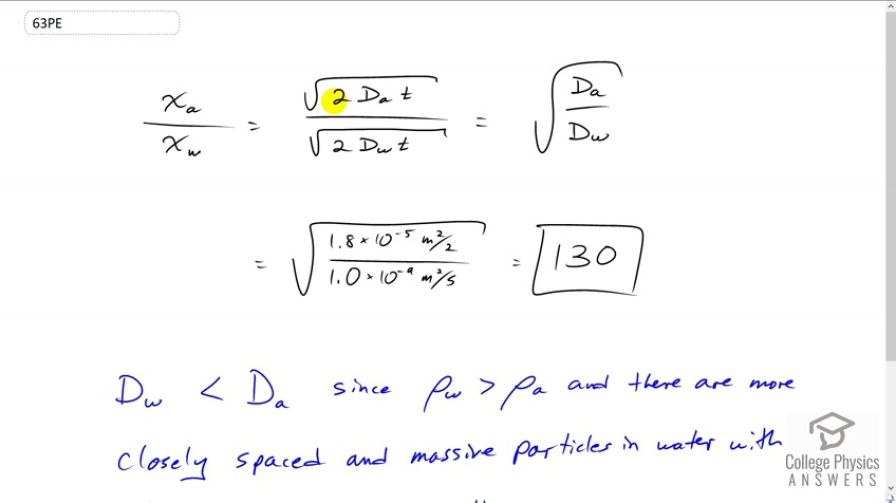
Final Answer
The diffusion constant of oxygen in water is less than that of oxygen in air since the density of water is so much greater than the density of air. This higher density of water means the water particles are much closer together, which results in many more collision between the oxygen and water, which impede the oxygen's progression.
Solution video
OpenStax College Physics for AP® Courses, Chapter 12, Problem 63 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. The ratio of the average distance that Oxygen will travel in air to the distance it will travel in water is the square root of two times this diffusion constant of Oxygen and air times time divided by square root of two times the diffusion constant of Oxygen and water times time. So the t and the twos are cancel here and we end up to the square root of Da over Dw. And so we look these numbers in our data table here, that's 12.2 and this works out to 130. So Oxygen will travel an average 130 times farther when in air than it would in water. And this is because the density of water is much greatest than that of air and that's a reflection of the act that the water molecules are so much closer together than the air molecule are. And so there are more collisions between the Oxygen and the water molecules because the water molecules is so much more close together. The water molecules are also, well more massive, actually that's not really true. H2O is actually lighter than Nitrogen for example, in the air which exists as a diatomic molecule in two. So actually air has molecules that are more massive than water molecules. And this is why when there's more water vapor in the air, the air pressure decreases because the water displaces some of the air molecules and since water molecules are less massive than air molecules that decreases the overall weight or pressure of the air. And so, low pressure means that rain is coming because there's more water in the air. But this is to say that definitely it's to do with greater density of water because of particles are so much closer in this liquid compared to the air in which the particles are spaced farther apart and so the Oxygen will be colliding more often with particles in the water. And so it's going to go more slowly.
