Question
Suppose hydrogen and oxygen are diffusing through air. A small amount of each is released simultaneously. How much time passes before the hydrogen is 1.00 s ahead of the oxygen? Such differences in arrival times are used as an analytical tool in gas chromatography.
Final Answer
0.39 s
Solution video
OpenStax College Physics for AP® Courses, Chapter 12, Problem 66 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
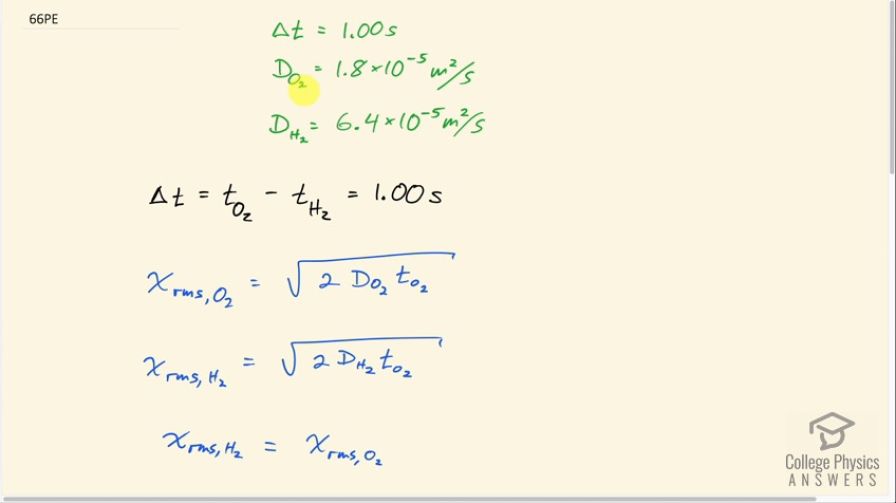
This is College Physics Answers with Shaun Dychko. Some oxygen and some hydrogen are released at the same time in the air, and they will travel such that they will arrive at some position one second apart. So the hydrogen will arrive at this position first. And this is the point of release here. And then this is the point of arrival. And the hydrogen will arrive here first and then the oxygen will arrive here one second later. And so the purpose in drawing this diagram here is to show that the RMS distance for the hydrogen and for the oxygen are the same. So they traveling the same distance, but they're arriving at different times, one second apart. So the difference between the time the oxygen takes, which will be longer and the time that hydrogen takes is one second. And we know the oxygen will take longer because it's diffusion constant is smaller. So in any given amount of time, it'll travel less distance. So here are the diffusion constants for oxygen and hydrogen, 1.8 times 10 to the minus five meters squared per second for oxygen and 6.4 times 10 to the minus five meter squared per second for hydrogen. And we write those things down here. And then we have this expression for the RMS distance, for oxygen is square root two times oxygen’s diffusion constant multiplied by the time for the oxygen to travel. And then the same corresponding formula for hydrogen. And these distances are equal. And so we equate these formulas then and then square both sides. And we have this line here. Two times the diffusion constant of hydrogen multiplied by the time for hydrogen to travel equals two times diffusion constant for oxygen multiplied by the time for oxygen to travel. And this can be replaced because we know since the difference in time is one second. That means the time for the oxygen is one second plus the time for the hydrogen. And so we can make a substitution then in place of the time for the oxygen. And now we have an expression with only one unknown, which is the time for the hydrogen to travel, which is what we're gonna solve. So we distribute this diffusion constant into the brackets. So it's D O two times one second plus D O two times time hydrogen. And then subtract this term from both sides and we end up with this line here and then we can factor out the time for the hydrogen to travel from both these terms and then we have this bracket and then we can divide both sides by that bracket and we end up with this line here. The time for the hydrogen to travel is the diffusion constant of oxygen times one second multiplied by the difference between the hydrogen and the oxygen diffusion constants. So that's 1.8 times 10 to the minus five meters squared per second times one second divided by 6.4 times 10 to the minus five meters squared per second, minus 1.8 times 10 to the minus five meters squared per second, which is 0.39 seconds. So after 0.39 seconds, the hydrogen will have reached a position such that the oxygen will take one more second to get there after.
