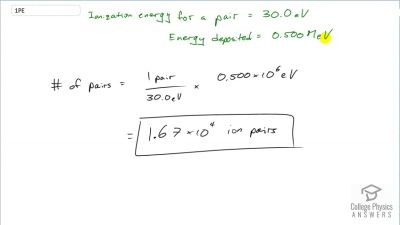
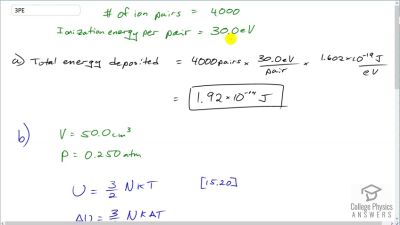
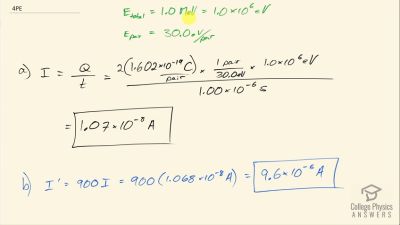
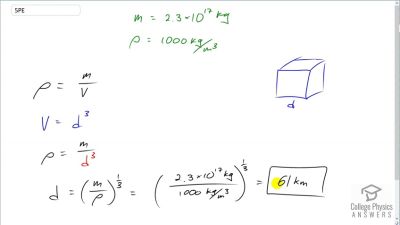
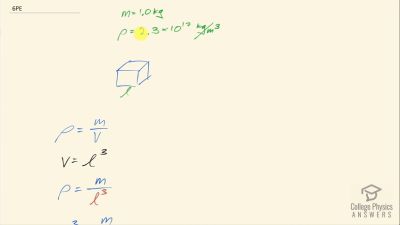
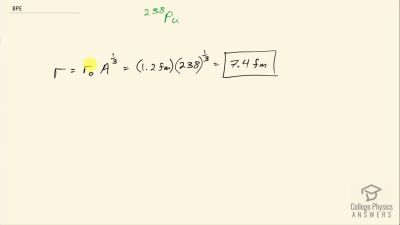
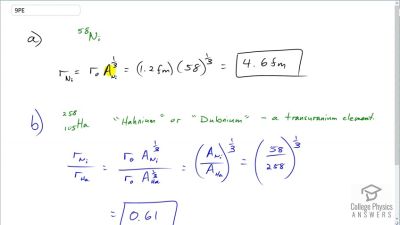
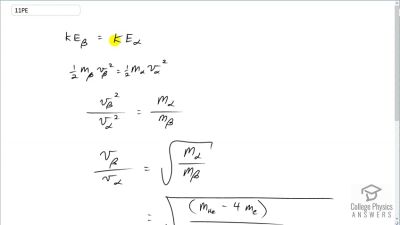
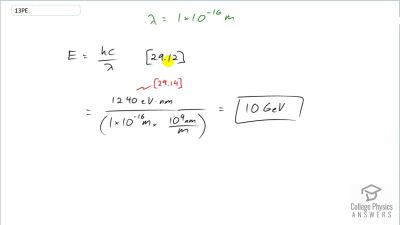
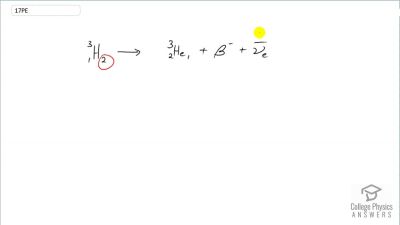
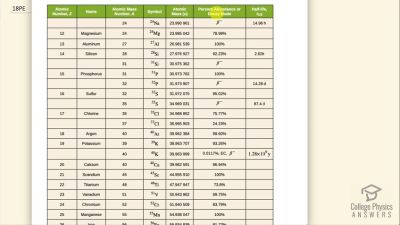
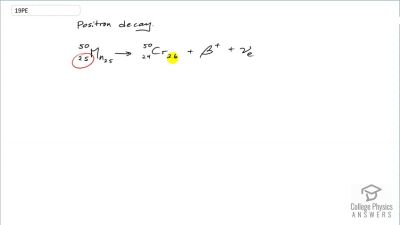
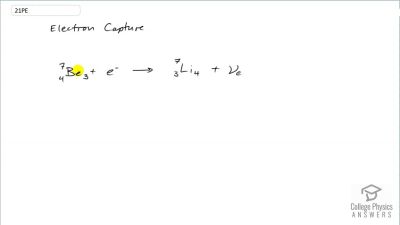
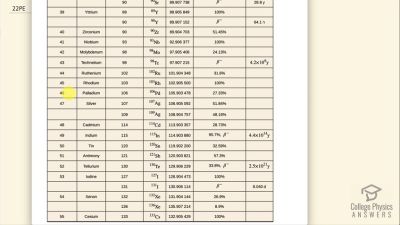
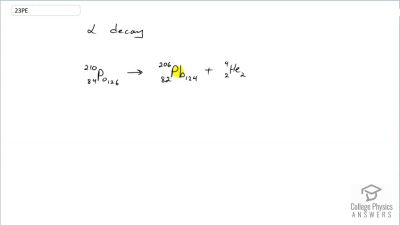
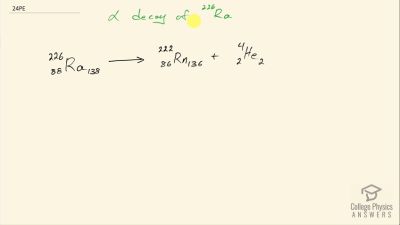
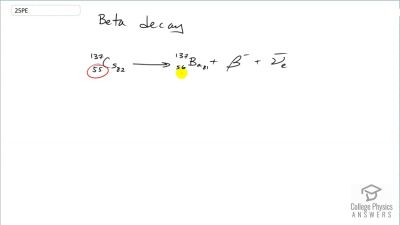
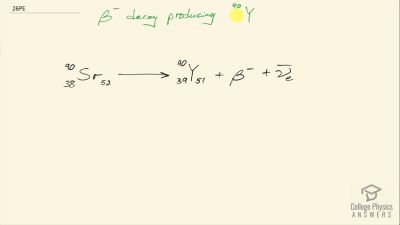
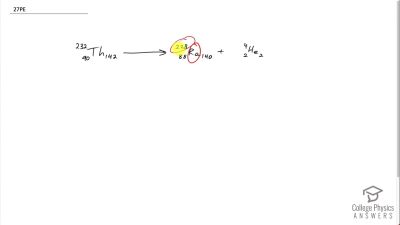
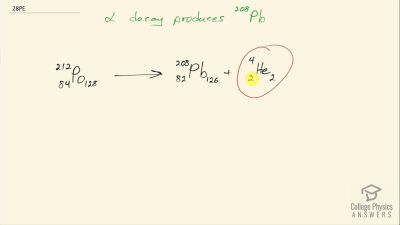
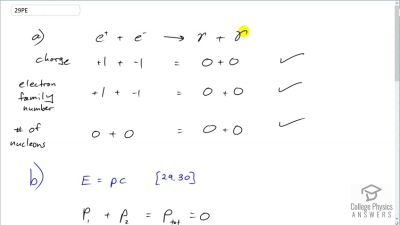
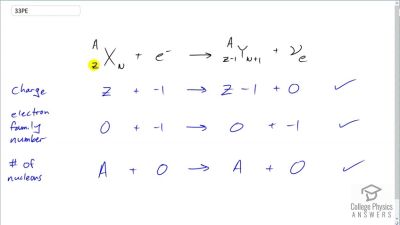
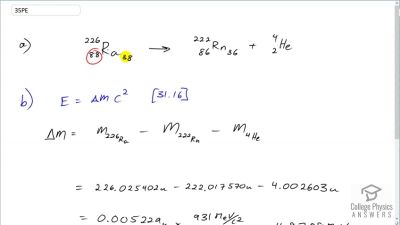
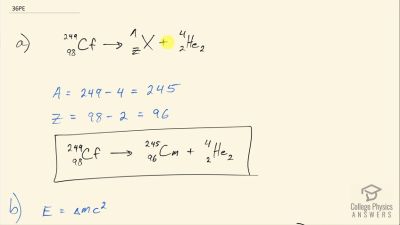
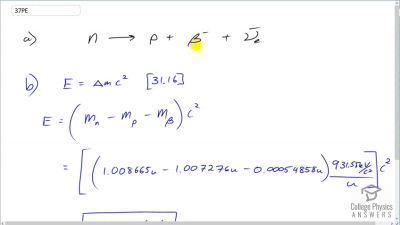
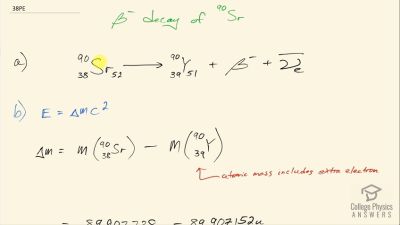
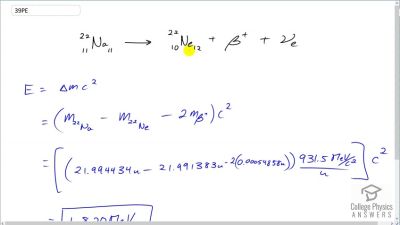
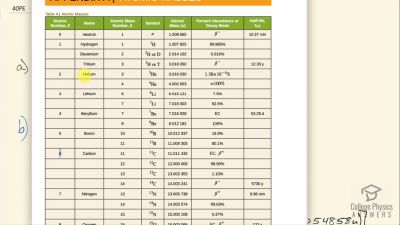
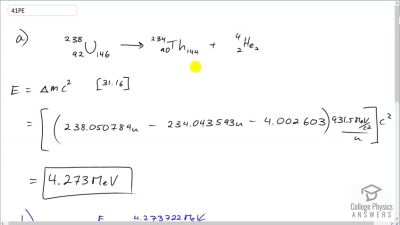
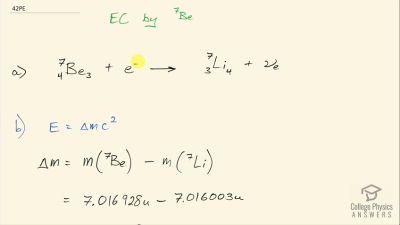
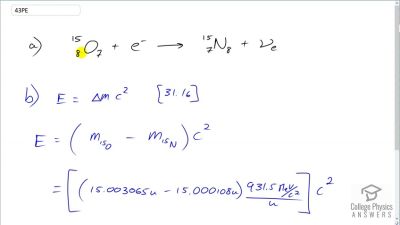
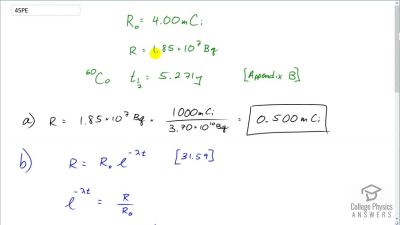
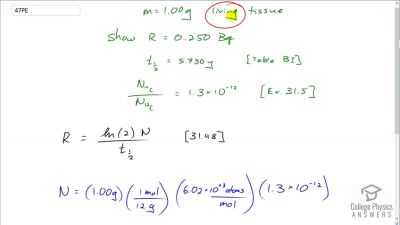
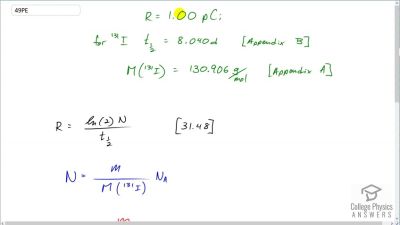
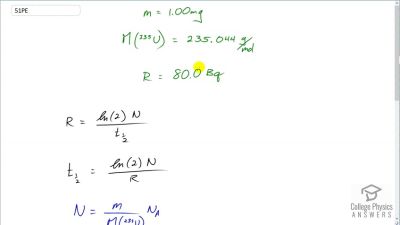
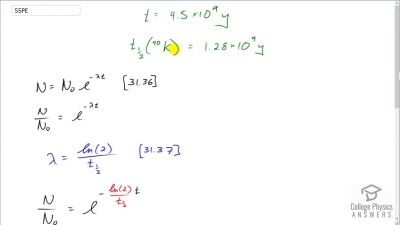
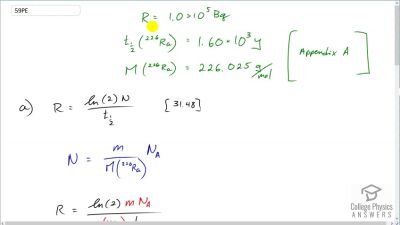
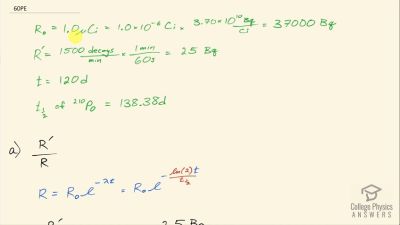
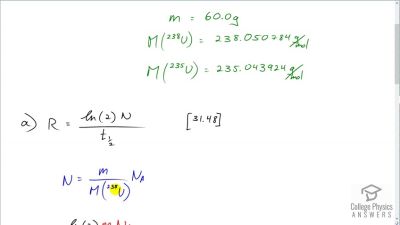
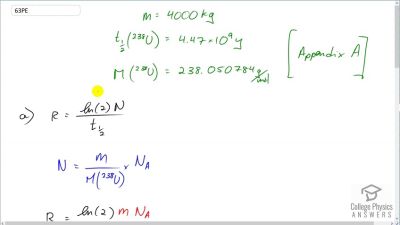
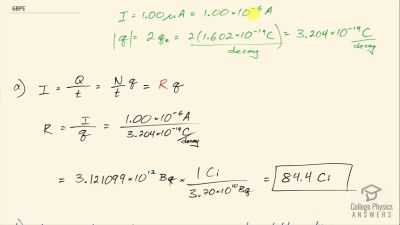
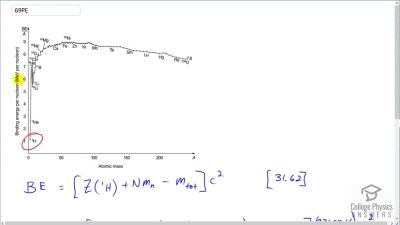
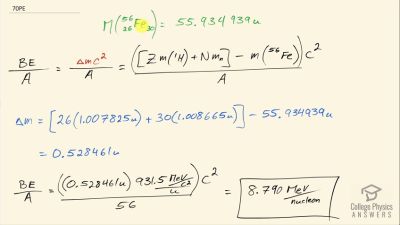
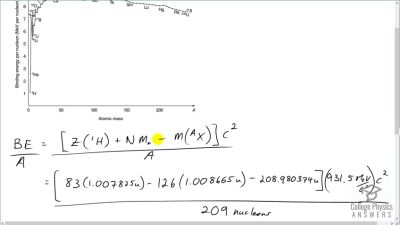
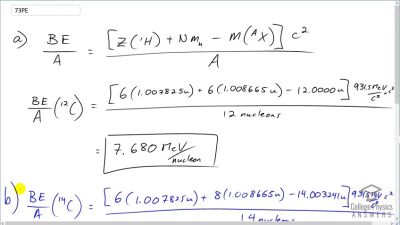
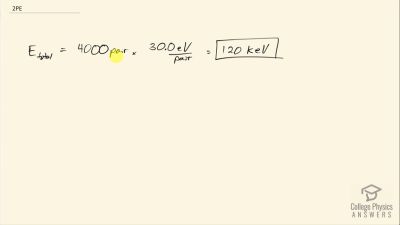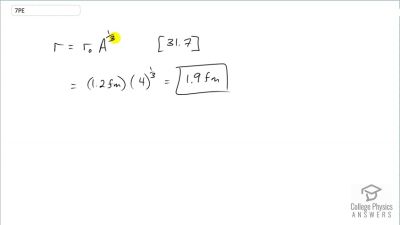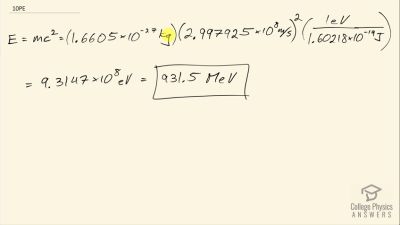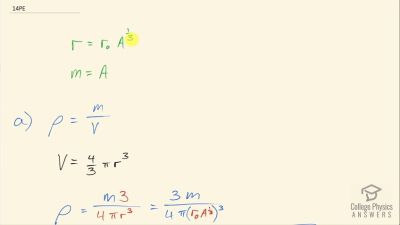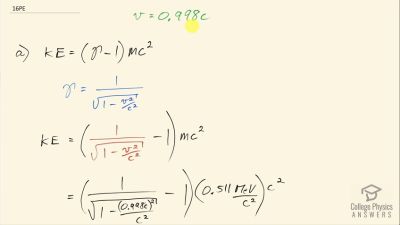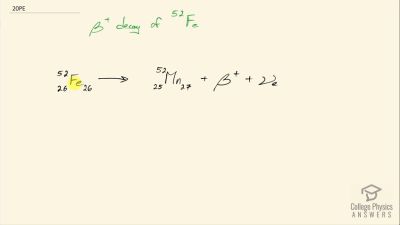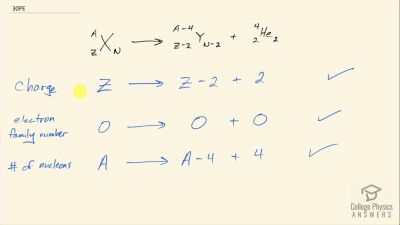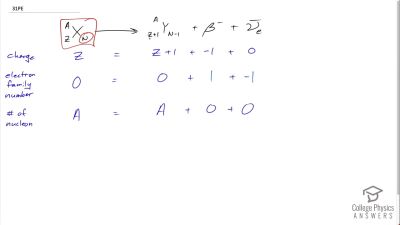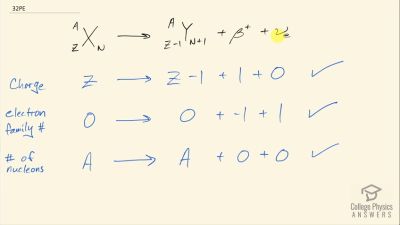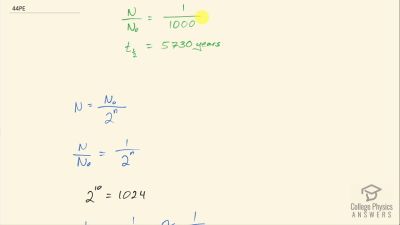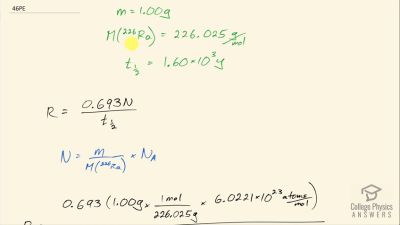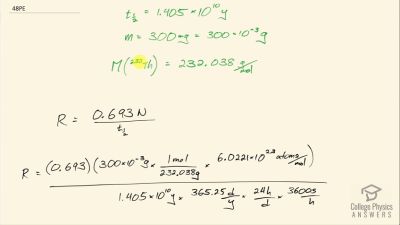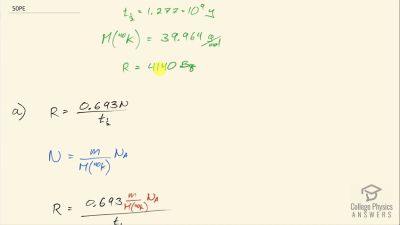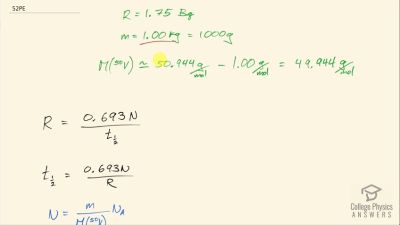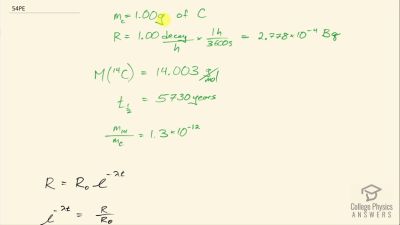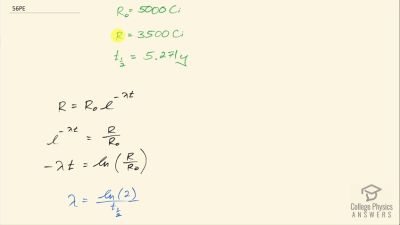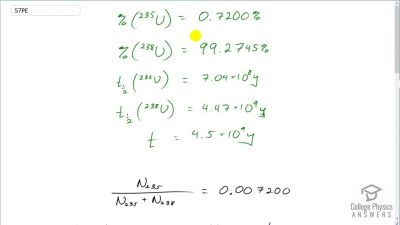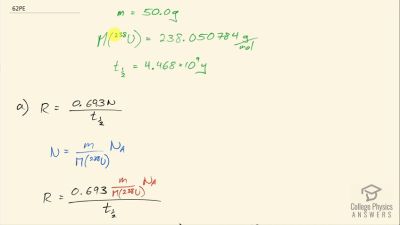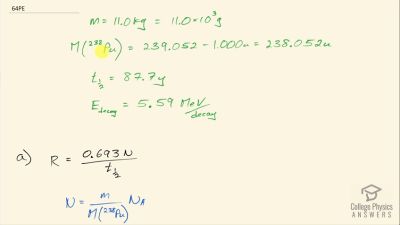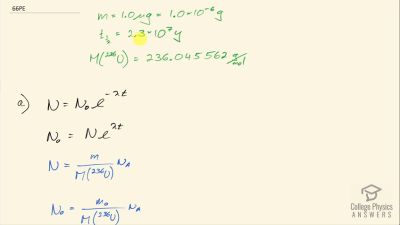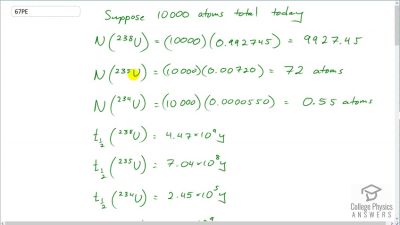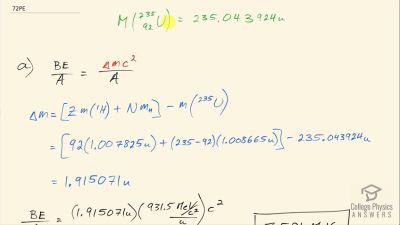When an electron and positron annihilate, both their masses are destroyed, creating two equal energy photons to preserve momentum. (a) Confirm that the annihilation equation
e++e−→γ+γ conserves charge, electron family number, and total number of nucleons. To do this, identify the values of each before and after the annihilation. (b) Find the energy of each
γ ray, assuming the electron and positron are initially nearly at rest. (c) Explain why the two
γ rays travel in exactly opposite directions if the center of mass of the electron-positron system is initially at rest.